Research Activities in Khani Laboratory of Electrochemistry and Materials Science
Khani’s work spans a diverse array of interdisciplinary projects in materials science and engineering, including electrochemical energy storage, sensors, electrocatalysis, electroplating, scanning electrochemical microscopy, and chemometrics. The current focus of research in the Khani Laboratory is on the design and synthesis of polymer and inorganic fast ion-conducting solid-state and quasi-solid-state electrolytes, polymer-based cathodes, electrocatalysts, and supercapacitors. We also conduct thermal, structural, and safety characterization of next-generation batteries. Our current projects include:
Solid-State Lithium-Metal Batteries
Our main research focus is dedicated to advancing solid-state batteries with a Li+-conducting solid-state electrolyte and lithium-metal anode, which promise higher energy density and enhanced safety compared to traditional lithium-ion batteries with carbon anode and flammable liquid electrolyte. We focus on developing and optimizing different types of solid-state electrolytes to overcome current limitations in solid-state batteries.
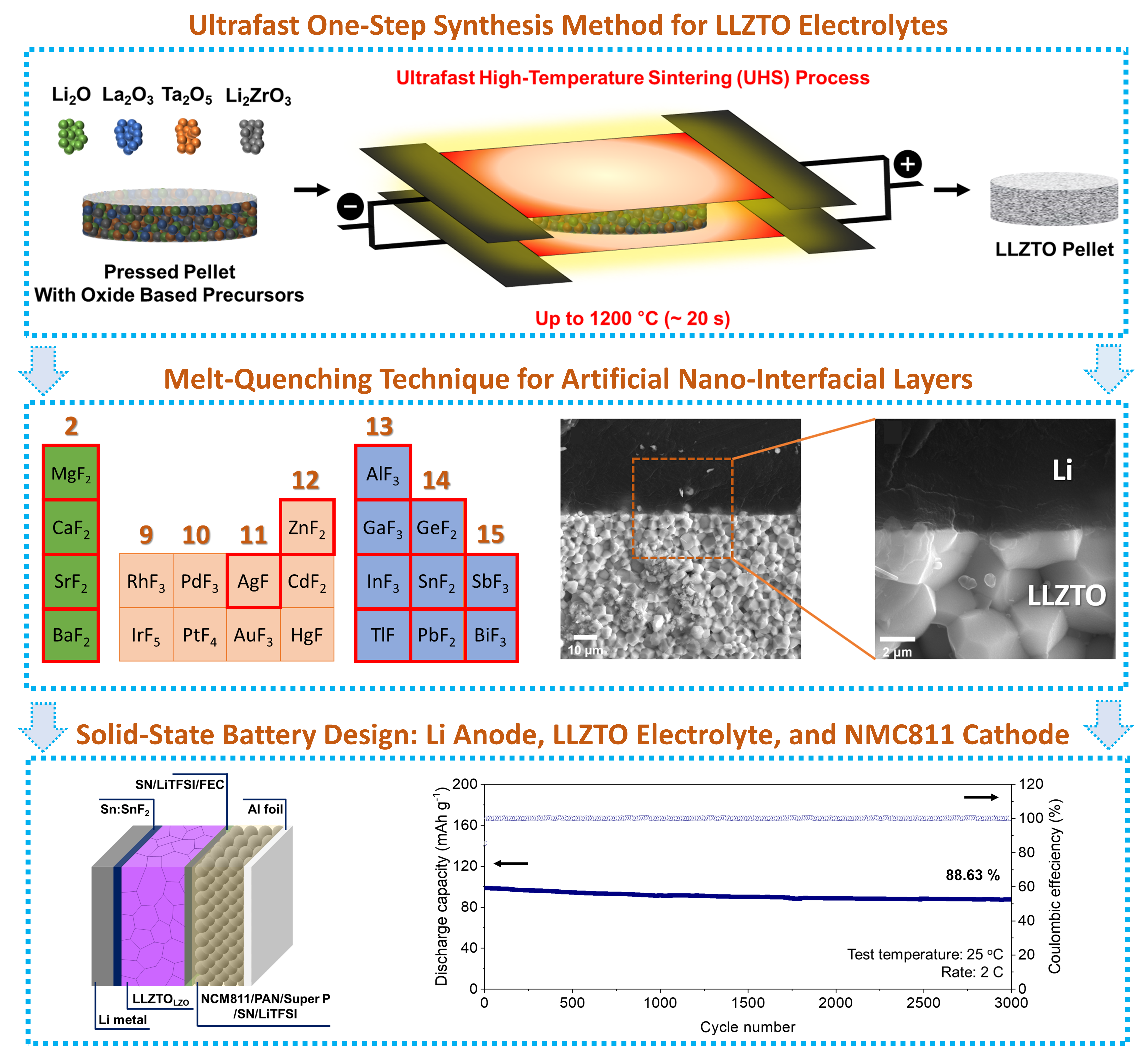
Inorganic Solid-State Electrolyte: Our interest in inorganic solid electrolytes centers around oxide-based solid-state electrolytes, such as garnet-type and NASICON-type oxides, due to their superior anodic and cathodic stability, high ionic conductivity, mechanical strength, and thermal stability. Our research involves developing new materials and synthesis methods to increase Li+ ion conductivity, reduce synthesis costs, and improve the interface chemistry between the solid electrolyte and both the cathode and lithium-metal anode, with the ultimate goal of improving battery performance and longevity.
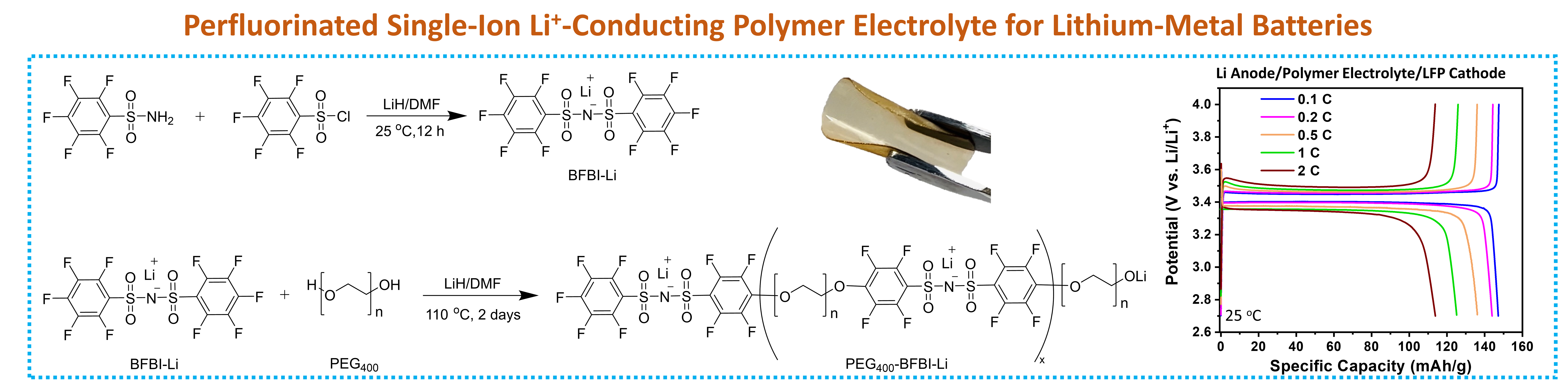
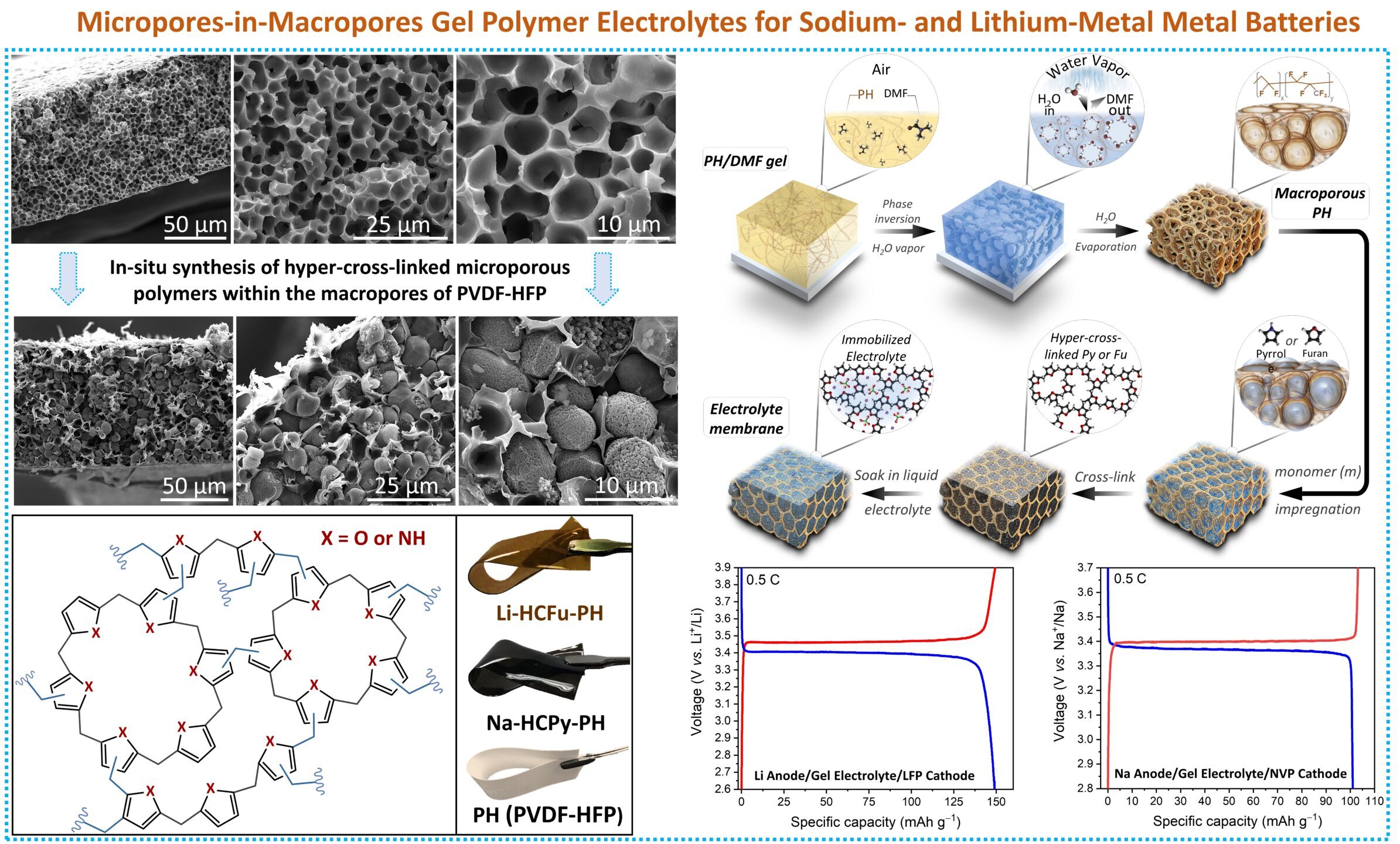
Polymer Electrolyte: We design and synthesize fast Li+-conducting polymer electrolytes, including polyethylene oxide-perfluorinated electrolytes, single-ion-conducting polymer electrolytes, and quasi-polymer electrolytes. Our polymer design strategy focuses on achieving a polymer electrolyte with high room-temperature Li+ conductivity (e.g., 10-3 S/cm), high interface stability with NMC cathodes, and high mechanical strength. A key advantage of polymer electrolytes is their high flexibility, making them compatible with current lithium-ion battery production processes.
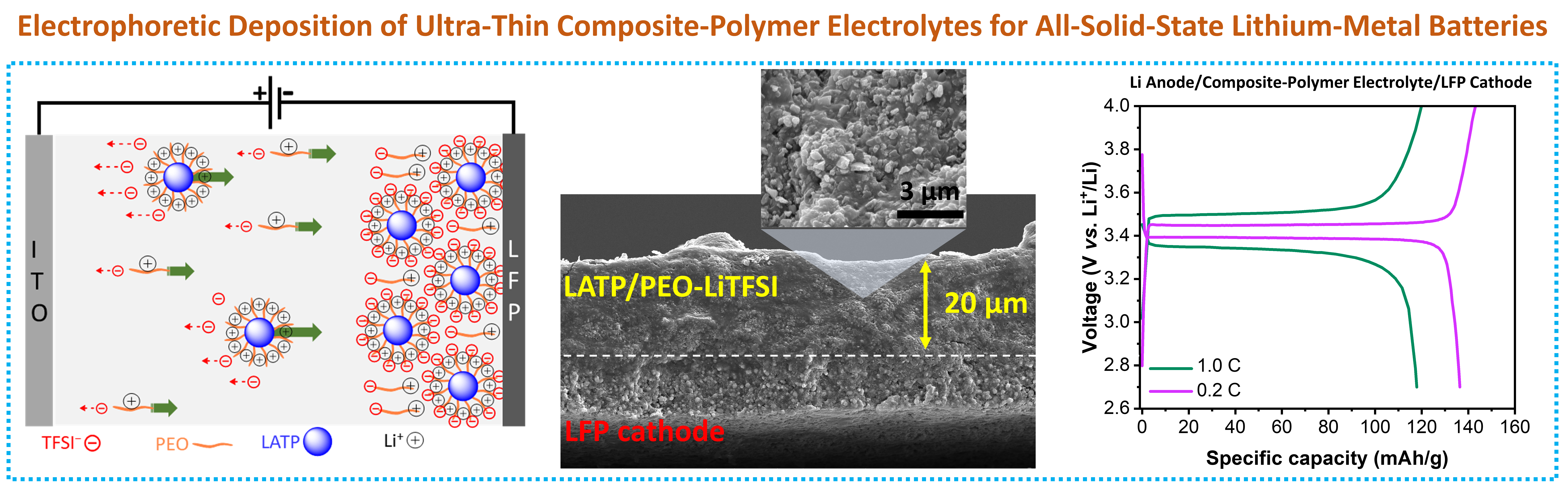
Composite-Polymer Electrolyte: We work on composite-polymer electrolytes to combine the benefits of inorganic fillers (e.g. high Li+ conductivity of 10-3-10-4 S/cm and high Young’s modulus of 100-150 GPa) and polymer matrices (high film flexibility, processability, and high electrolyte/electrode interface contact). By incorporating nanoparticles or nanofibers of inorganic fillers into polymer electrolytes, we aim to boost ionic conductivity, mechanical robustness, and interfacial stability. This hybrid approach seeks to overcome the shortcomings of individual electrolyte systems in all solid-state batteries.
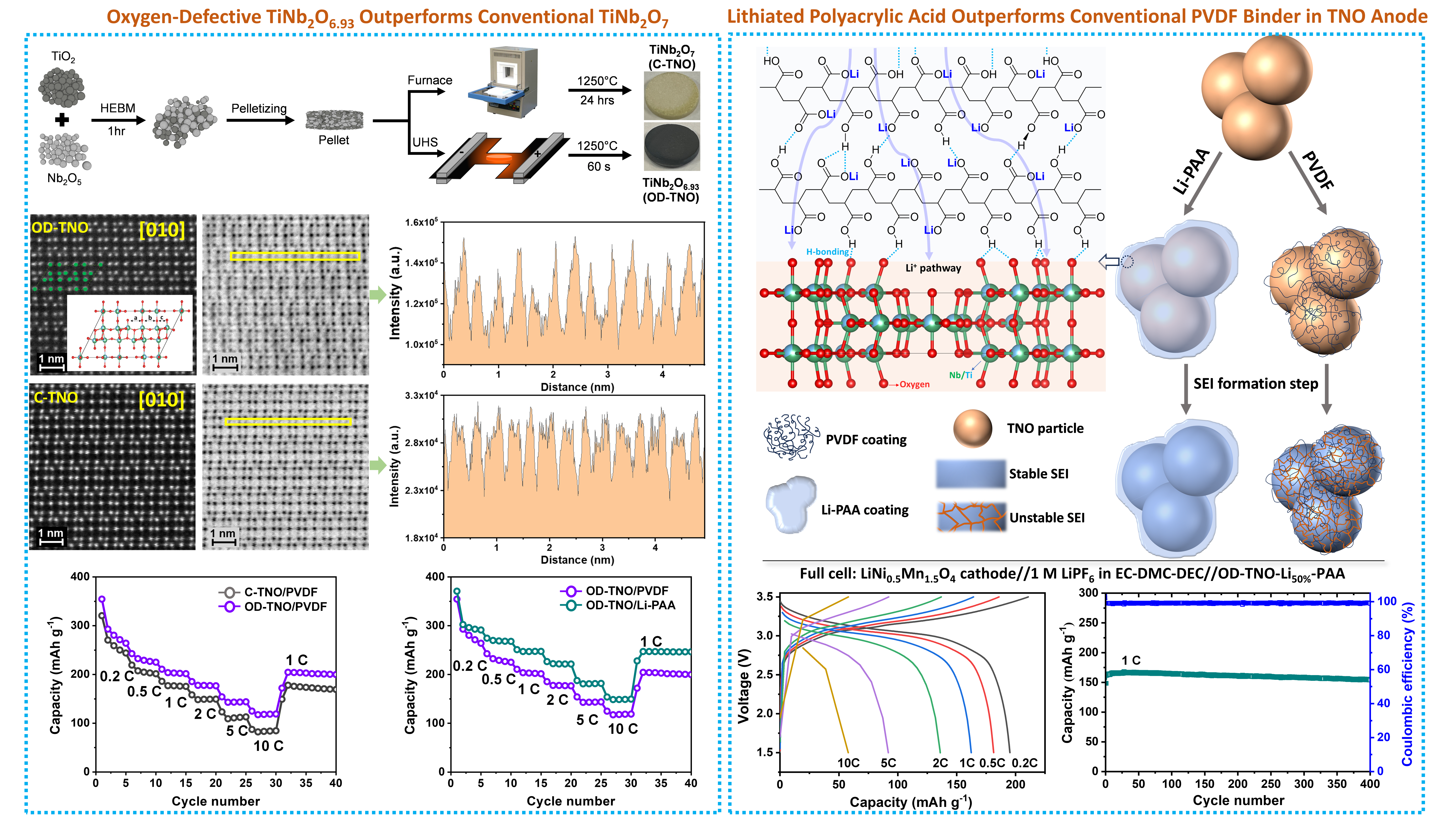
Metal Oxide Anode for Ultrafast-Charging Applications
We work on different metal oxide anodes, including TiNb2O7, Nb2O5, and V2O5, for fast-charging and low-temperature applications, where traditional lithium-ion batteries with carbon anodes have significant limitations. The goal is to increase the intrinsic ionic and electronic properties of these metal oxides and improve the interface of liquid electrolyte with these metal-oxide anodes to enable safe, fast-charging applications (e.g., 10C, 6 minutes). We use various electrochemical and computational tools to understand the ionic and electronic conduction mechanisms in these materials, allowing us to tune their chemistry for target applications.
Thermal and Safety Characterization of Next Generation Batteries
This research focuses on the thermal, structural, and gas release behaviors of next-generation battery systems under normal and abusive thermal and electrochemical conditions. To achieve these goals, we employ a combination of advanced tools, including operando battery-mass spectroscopy, operando battery-Raman spectroscopy, operando battery-ultrasonic imaging, differential electrochemical mass spectrometry (DEMS), and differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) coupled with mass spectrometry (MS) and Fourier transform infrared spectroscopy (FTIR). Our goal is to establish a comprehensive materials-level and cell-level understanding of failure mechanisms, reaction pathways, failure modes and effects, and to develop revised testing standards, new capabilities, and tools to help de-risk the adoption of next-generation cells.
Electrocatalysis
In this area, we aim to investigate the structure-activity relationships in electrocatalyst materials using advanced operando electrochemical and surface characterization techniques such as operando surface-enhanced Raman spectroscopy and scanning electrochemical microscopy. Our goal is to delve into the mechanisms of electrochemical reactions—such as carbon dioxide reduction (CO2RR), oxygen evolution reaction (OER), oxygen reduction reaction (ORR), and hydrogen evolution reaction (HER)—on metal-based electrocatalysts, ultimately using this information to enhance activities and selectivities.
Sponsors