Current projects in the laboratory address the following questions:
- Do proteases, expressed in the bladder wall after an acute spinal cord injury, drive aberrant remodeling that culminates in bladder dysfunction?
- Can cell-type specific neuromodulation improve bladder function outcomes in a model of spinal cord injury?
- Do infiltrating polymorphonuclear leukocytes have divergent roles in modulating recovery after injury to the developing brain?
- Is the gyrencephalic brain a better preclinical model to study how the human brain responds to a traumatic injury
- Does early maternal stress influence the extent of recovery after a traumatic brain injury?
Project 1: Defining the early substrates that support recovery of bladder function after spinal cord injury.
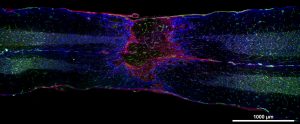
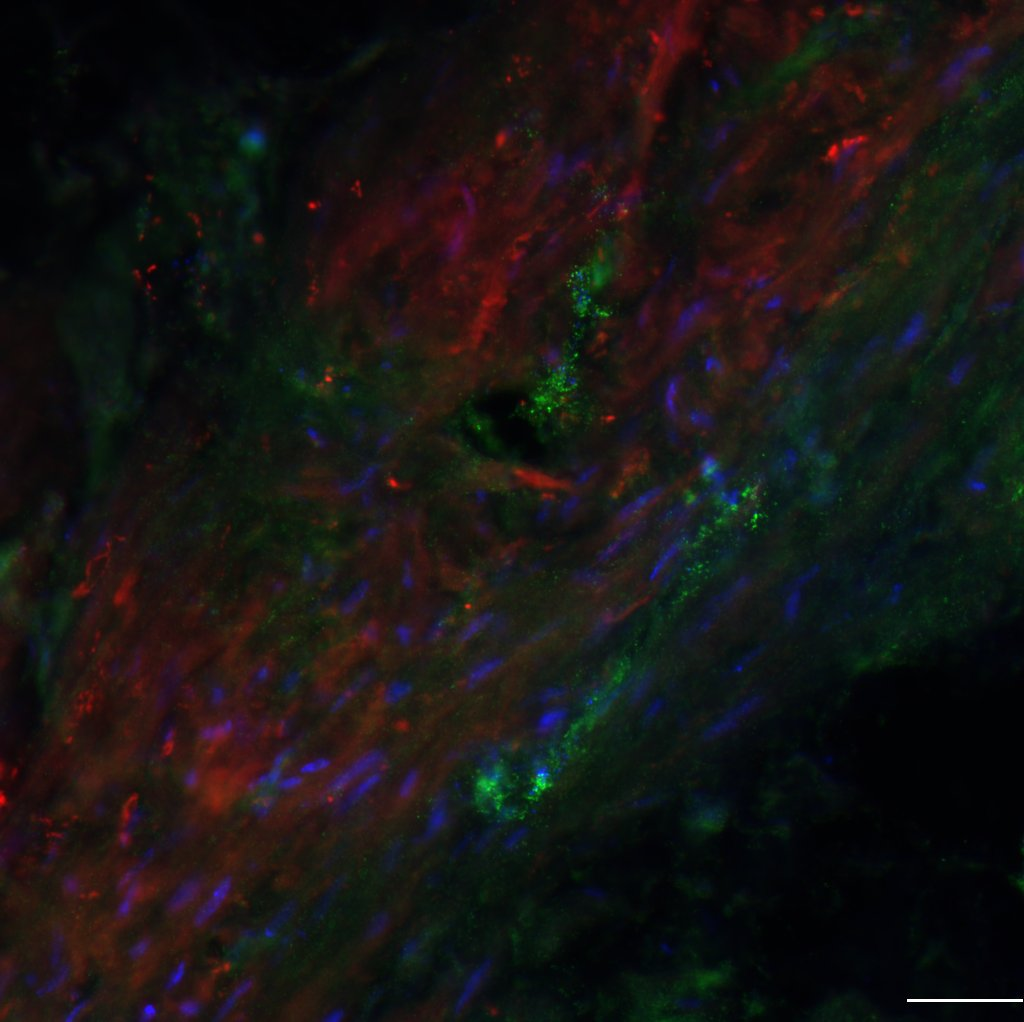
A longitudinal section revealing the lesion epicenter that is surrounded by a glial scar (pink) and vascular structures (green), residing both within the epicenter and along the axis of the spinal cord. Our recent preliminary studies reveal the importance of matrix metalloproteinases (MMPs) in modulating recovery of bladder function after spinal cord injury. Early systemic blockade of MMPS results in improved bladder function. We are now addressing the mechanisms underlying this improvement, focusing on the role of MMPs, expressed acutely in the bladder wall, on aberrant remodeling of the smooth muscle that facilitates bladder contraction.
Check out this video of the 3-dimensional vasculature within the injured spinal cord, as viewed by 2-photon miscroscopy in cleared sections !
Project 2: Applying optogenetic and sono-optogenetic technology to modulate bladder function in a model of spinal cord injury
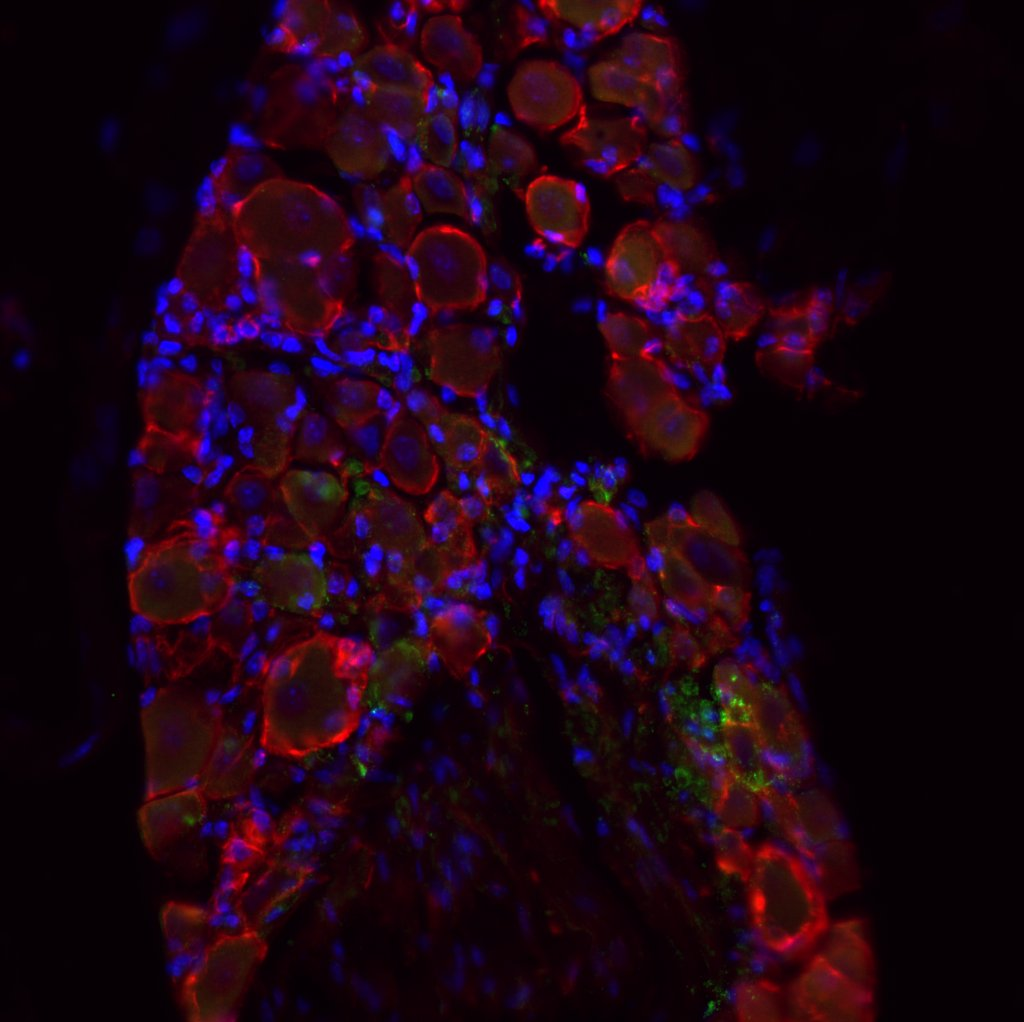
The left image shows the bladder and the right shows the dorsal root ganglion (DRG), both expressing opsins, with βIII-Tubulin in red, GFP in green, and DAPI in blue; scale bar 50 µm. Optogenetics is a cell-type specific tool that uses light to modulate specific neurons in the central and peripheral nervous system. This project aims to apply optogenetics to contract the bladder and inhibit the external urethral sphincter to facilitate voiding in injured mice who otherwise are not able to void. Target neurons in the lower urinary tract need to be infected with an opsin-carrying virus so that specific pathways between the spinal cord and lower urinary tract become light-sensitive. We plan to use externally implanted sources of light and novel mechanoluminescent nanoparticles that can emit light in response to focused ultrasound.
Project 3. Defining the pathways for leukocyte recruitment into the injured young brain

In vivo 2-photon imaging of the superficial vessels in the murine brain after traumatic injury at a young age showing vascular leakage (red) in left panel and infiltrating leukocytes (round bright green structures) in right panel. This project will elucidate the role of activated polymorphonuclear leukocytes (PMNs) in mediating barrier disruption and local tissue destruction, if their phenotypic diversity is governed by the local environment and if selective temporal modulation of PMNs will enhance neurological recovery.
Project 4. Defining the substrates underlying recovery after traumatic injury to the developing ferret brain
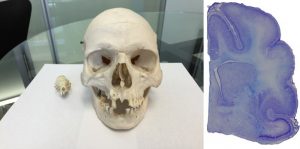
Left panel: Size comparison between the ferret and human skulls. Right panel: The ferret brain is gyrencephalic, a feature that is likewise seen in the human brain. The lab is developing a ferret, closed-head model of mild traumatic brain injury where the focus will be on defining the earliest indices of injury across cell types/regions and the longer-term changes in white matter integrity, glial reactivity, and behavioral function.
Project 5: Early determinants of lesion expansion after traumatic injury to the developing brain.
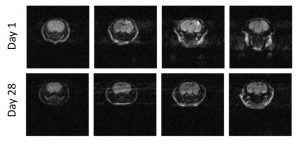
Diffusion weighted imaging of the murine brain after traumatic injury to the right frontoparietal cortex at postnatal day 21. Our previous work has shown that the cortical injury increases with time, resulting in a long-term defect in the cortical mantle. The intent now is to determine if early recruitment of leukocytes to the injured brain is a key determinant in the evolution of the cortical injury.
Project 6: Impact of early life maternal stress on neurological recovery after traumatic injury to the developing brain.
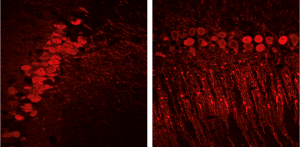
Immunolocalization PCP4 in CA2 hippocampal neurons and Purkinje cells in the cerebellum. While the hippocampus has been widely studied in the injured adult brain, few studies have examined its vulnerability after injury at a young age. Our recent published studies have revealed that injury at a young age results in increased susceptibility to seizure activity, a finding which may at least in part account for the loss of neurons in the hippocampus over time. In this project we will define how a traumatic brain injury effects the structural/functional integrity of CA1, CA2, and CA3 by taking advantage of PCP4 which is a distinct marker for CA2. We will further determine if maternal stress, prior to a traumatic injury at a young age, influences hippocampal structure and function and worsens long-term cognitive and social functions.