- Characterizing macrophage repolarization by engulfed apoptotic cells in regenerative medicine
Summary: Macrophages that have engulfed apoptotic cells tend to polarize toward an anti-inflammatory (M2) phenotype over a pro-inflammatory (M1) phenotype. Our lab is interested in which characteristics of apoptotic cells cause this shift. We also utilize the anti-inflammatory nature of apoptotic cells to produce nanoparticle systems to aid in the calming of inflammation for regenerative medicine.
Engineering Nanoparticles to Control Macrophage Phenotype
Project One
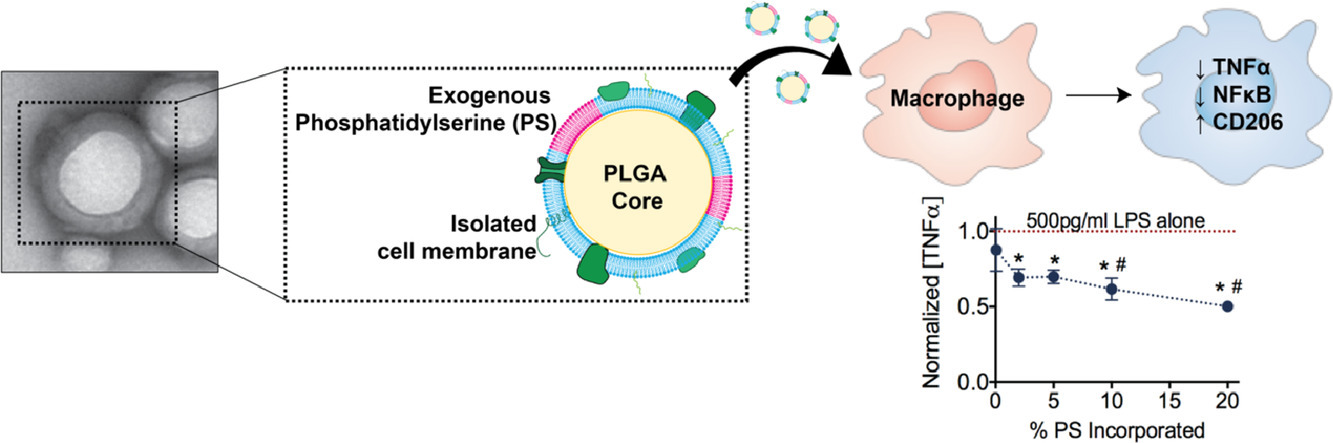
Current anti-inflammatory drugs have significant drawbacks, including variable response rates and off-target effects. Here, we have developed an apoptotic-body inspired nanoparticle to modulate inflammatory macrophage phenotype. This polymeric nanoparticle is coated with phosphatidylserine-supplemented cell plasma membrane to mimic the anti-inflammatory effect of apoptotic cell engulfment. Nanoparticle delivery reduces inflammatory cytokine production and promotes an anti-inflammatory phenotypic macrophage shift. The capacity of these nanoparticles to help resolve macrophage-mediated inflammation may be a useful tool to study macrophage-apoptotic cell interactions, the role of macrophages in inflammatory diseases, and in the design of anti-inflammatory therapeutics.
Nanoparticle Size, Stiffness and Surface Properties Impact Uptake
Project Two
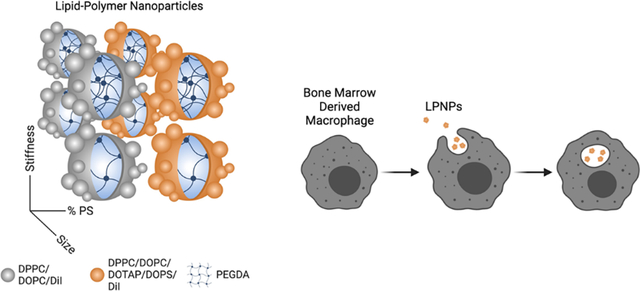
In this work, we created an array of eight lipid-polymer nanoparticles (LPNPs) that are small/large, soft/stiff, and with/without PS. These LPNPs consist of a polymer core to impart physical attributes surrounded by a lipid coating to aid in cellular interactions. When given to bone marrow-derived macrophages, LPNPs that were large, stiff, and contained phosphatidylserine (PS) were taken up significantly more than any other formulation, demonstrating the importance of all three physicochemical characteristics on endocytosis and indicating that these characteristics work better together than alone.
