- Design of self-assembling, peptide-based hydrogels for drug delivery and tissue engineering
Summary: Scaffolds play essential roles in drug delivery and tissue engineering. They not only provide the architecture to deliver therapeutics and to support cells, but also physical cues for cell behavior, including attachment, proliferation, and gene expression. Superior scaffolds have a variety of characteristics in common: they have a physical structure similar to the extracellular matrix (ECM); they are robust enough to support either drug loading and controlled release or cell attachment and cellular expansion; they are highly hydrated at physiological conditions; they are diffusive enough to allow nutrients, growth factors, or bioactive molecules to reach cells in addition to facilitating the removal of cellular wastes; and they have built-in degradability that allows for easy clearance with nontoxic byproducts.
Protein-inspired, peptide hydrogels are promising for biomaterials applications. Hydrogels made from self-assembling peptides have significant potential in drug delivery and tissue engineering because they provide a highly hydrated, ECM-like environment with inherent biocompatibility and straightforward tunability due to the nature and character of amino acids; such constructs therefore have characteristics that mimic the natural environment and can be readily designed for specific purposes. Small molecule, self-assembling peptides are a promising new field in scaffold-design research.
Hydrogels for 3D Cell Cultures
Project One
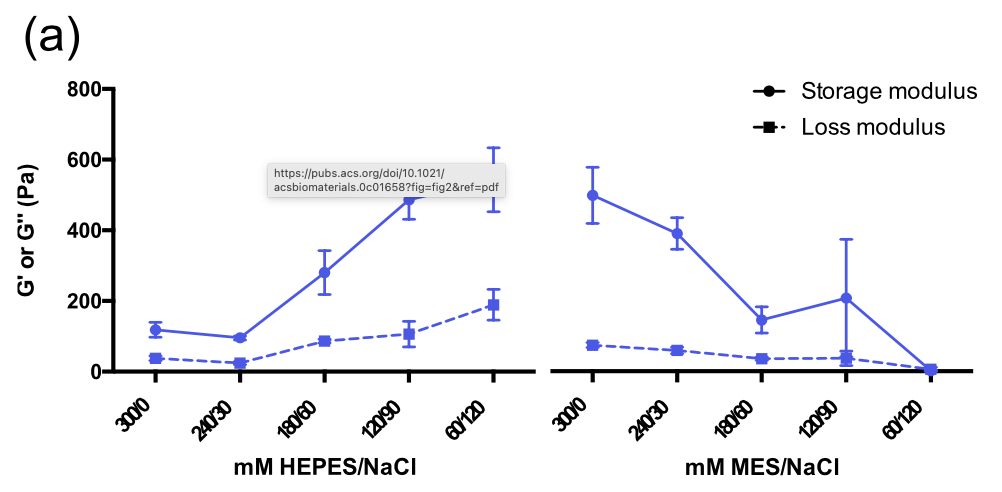
We are investigating novel nucleopeptide hydrogel scaffolds to mimic a three-dimensional (3D) environment. Current 3D hydrogels for cell culture, like Matrigel, are widely used but inherently flawed due to their biological origin. By constructing a synthetic peptide sequence which undergoes gelation in physiological conditions we aim to develop a system that is modular and reproducible. Herein, we have assesed numerous combinations of short amino acid sequences to create a hydrogel capable of supporting cells in a 3D environment. Diverging from traditional solid-phase peptide synthesis, we have substituted the controversial Fmoc molecule with biocompatible nucleobases.
Carrier for miRNA cargo
Project Two

We further aim to capitalize on our unique nucleopeptides by analyzing their capacity to act as a vector for nucelic acids, specifically microRNA (miRNA). Our material will inherently intercalate with the cargo to facilitate entry into cells and improve genetic uptake by overcoming at least one of the biological barriers associated with therapeutic delivery.