Mechanisms and Pathways of Plasmalemma Repair
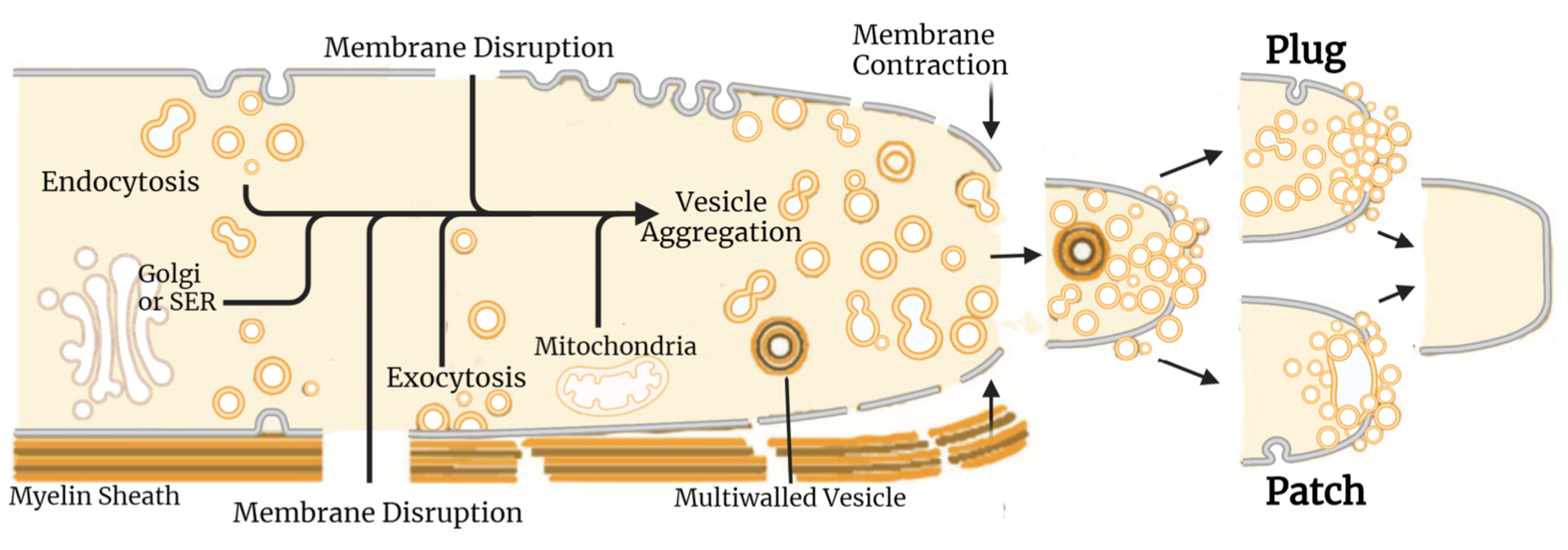
The four papers listed below were the first to show that plasmalemmal lesions in all eukaryotic cells are sealed by accumulation of vesicles, endocytosis that require calcium calpain, and other substances also used in exocytosis, Golgi trafficking, and synaptic release. Our laboratory has been the leader in determining the cellular and biochemical pathways and mechanism for such sealing – and to show that PEG bypasses these natural pathways to directly induce plasmalemmal collapse and sealing, aka “PEG-sealing”. We have published about 10 papers documenting such phenomena and biochemical pathways and are still currently making an effort to better characterize them with more advanced microscopy. We have used these data to develop our well-specified sequence of bio-engineered solutions to successfully PEG-fuse transected PNS axons or ablated PNS axonal segments repaired by PEG-fused interposition autografts, isografts, or allografts.
- C.S. Spaeth, E.A. Boydston, L.A. Figard, A. Zuzek and G.D. Bittner. 2010. A model for sealing plasmalemmal damage in neurons and other eukaryotic cells. J. Neurosci. 30:15790-15800.
- C.S. Eddleman, M.L. Ballinger, M.E. Smyers, H.M.Fishman, G.D. Bittner (1998). Endocytotic formation of vesicles and other membranous structures induced by Ca2+ and axoplasmic injury. J Neurosci,18, 4029-4041.
- C.S. Eddleman, M.L. Ballinger, M.E., Smyers ,C.M. Godell, H.M., Fishman, G.D.Bittner (1997). Repair of plasmalemmal lesions by vesicles. PNAS, 94, 4745-4750.
- T.L. Krause, H.M. Fishman, M.L. Ballinger, G.D. Bittner (1994). Extent and mechanism of sealing by vesicles, often formed by exocytosis transected giant axons of squid and earthworms. J Neurosci, 14, 6638-6651.
PEG-Induced Axonal Fusion Repair
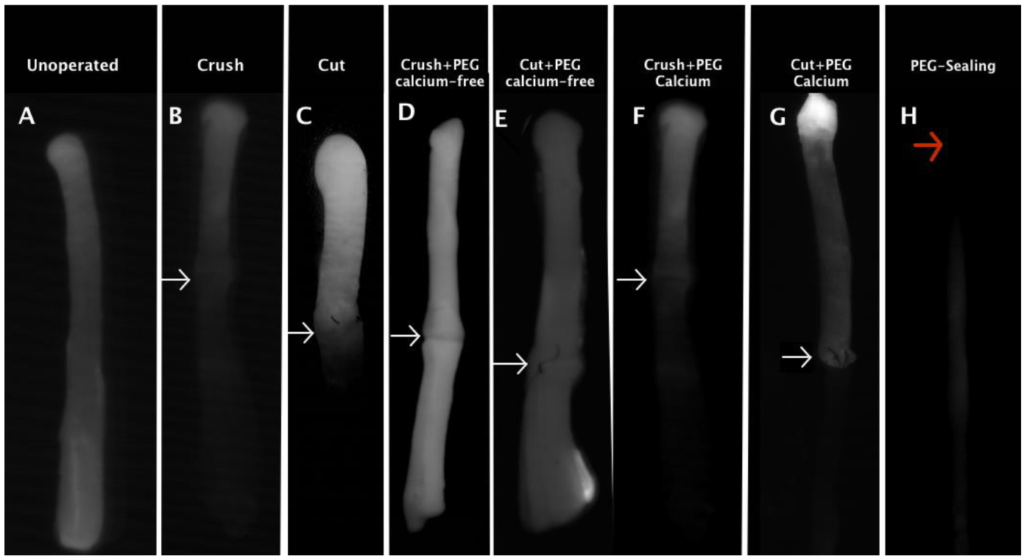
The four papers listed below were the first to show that PEG can be used to repair severed invertebrate giant axons ex vivo, then in vivo, then mammalian peripheral nerve axons and spinal tract axons ex vivo, then crushed or cut mammalian peripheral nerve axons in vivo in Ca2+-free or Ca2+-containing solutions, then ablated mammalian peripheral nerve axons in vivo in Ca2+-containing solutions. That is, from our papers listed below and our recent papers, we have demonstrated successful PEG-fusion of cut, crushed, or ablated mammalian axons mostly in a rat model of acute repair of mixed motor and sensory sciatic nerve. We have also demonstrated that our PEG-fusion technology is translatable to rabbit or guinea pig peripheral nerve axons and to rat or guinea pig spinal tract axons, as well as giant axons from three invertebrate phyla. We have also demonstrated that severed distal rat sensory and motor peripheral nerve axons can be viably maintained for 5-10 days by cooling or cyclosporine A injections, and that peripheral nerve axons chronically maintained for at least 3 days can be successfully PEG-fused as measured by compound action potential conduction through the lesion, including for chronically maintained interpositioned autografts or allografts. Considered together, these data strongly suggest that this PEG-fusion technology using micro-sutures and well-specified sequences of solutions is translatable to repair acutely or chronically (1-3d) severed nerves in animals from many different phyla and different mammals, at least for nerves up to 3 mm in diameter with gaps up to 10mm. This current PEG-fusion technology has in fact taken decades to develop with conceptual and practical contributions. We have published many papers in the last 10 years on this topic (see Publications) and are the leading laboratory in rapid repair of traumatic lesions to peripheral nerve axons. We have begun to apply this PEG-fusion technology to repair spinal cord injuries.
- D.C. Riley, G.D. Bittner, M.A. Mikesh, N.L. Cardwell, A.C. Pollins, C.L. Ghergherehchi, S.R. Bhupanapadu Sunkesula, T.N. Ha, B.T.D. Hall, A.D. Poon, M. Pyarali, R.B. Boyer, A.T. Mazal, N. Munoz, R.C. Trevino, T.Schallert, W.P. Thayer. 2015. Polyethylene glycol–fused allografts produce rapid behavioral recovery after ablating sciatic nerve segments. J Neurosci Res. 2015 Apr;93(4):572-83. doi: 10.1002/jnr.23514. Epub 2014 Nov 25.
- G.D. Bittner, C.P. Keating, J. R. Kane, J.M. Britt, C.S. Spaeth, J. D. Fan, A. Zuzek R.W. Wilcott, W. P. Thayer, J.M. Winograd, F. Gonzalez-Lima and T. Schallert. 2012. Rapid, effective and long-lasting behavioral recovery produced by microsutures, methylene blue and polyethylene glycol after complete cut of rat sciatic nerves. J Neurosci Res. 90:967-980.
- A.B.Lore, J.A.Hubbell, D.S. Bobb Jr., M.L.Ballinger, K.L. Loftin, , J.W.Smith, M.E. Smyers, H.D.Garcia and G.D. Bittner. 1999. Rapid induction of functional and morphological continuity between severed ends of mammalian or earthworm myelinated axons. J Neurosci. 19: 2442 – 2454.
- T.L. Krause and G.D. Bittner. 1990. Rapid morphological fusion of severed myelinated axons by polyethylene glycol. PNAS. 87:1471-1475.
