High-temperature hypersonic environments are simulated using a 50 kW inductively-coupled plasma (ICP) torch. The ICP torch is the key component of a high-temperature materials testing facility that was developed in collaboration with Prof. Philip Varghese. The ICP Torch, model APT-50, which includes the DC power supply, RF generator and torch head, was designed and built by Applied Plasma Technologies, Corp. The torch heats air to approximately 6000 to 7000 K with heat fluxes up to about 350 W/cm^2 when fitted with a converging nozzle. The torch operates continuously and exhausts to atmospheric conditions. Typical measurements that are made include surface temperature, material mass loss and shape change, optical and SEM microscopy, emission spectroscopy, laser-induced fluorescence of ablation products and Raman scattering to characterize the exit plume.
ICP torches are well-established devices for heating gases to temperatures of order 10,000 K. ICP torches use magnetic induction, rather than metal electrodes, for heating and so the heated gas is not contaminated by electrode material as with arcjets. This “clean gas” feature is particularly important when using the torch to study high-temperature chemistry. Furthermore, the heating is done over an extended volume and so the flow inside the torch tends to be close to thermochemical equilibrium, which means that testing takes place with known freestream conditions. These features make the ICP ideal for many purposes where a clean, well-characterized high-temperature flow is needed. The ICP works by passing an oscillating current through the induction coils that surround the gas to be heated. The oscillating current induces an oscillating magnetic field that permeates the medium and induces an oscillating electric field that accelerates free electrons and ions, which in turn collide with atoms and molecules causing further ionization and heating. Some source of electrons is needed to initiate the process, which can be accomplished by using a spark igniter.
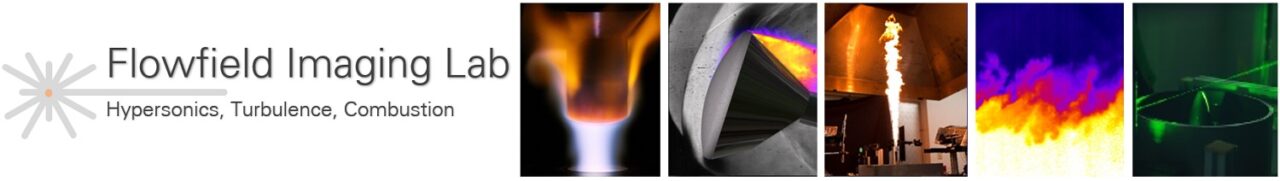
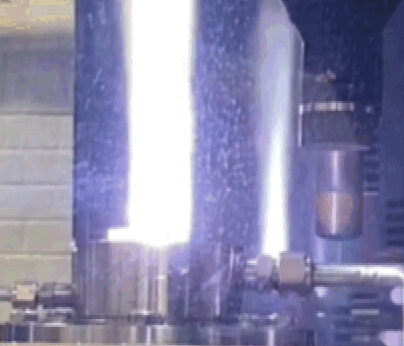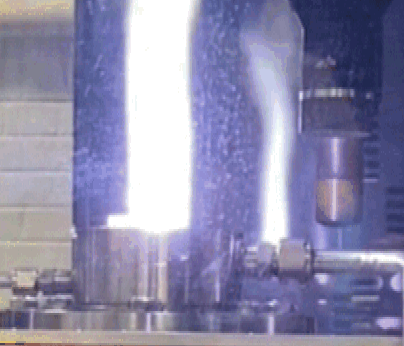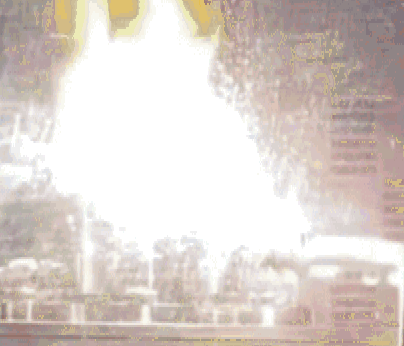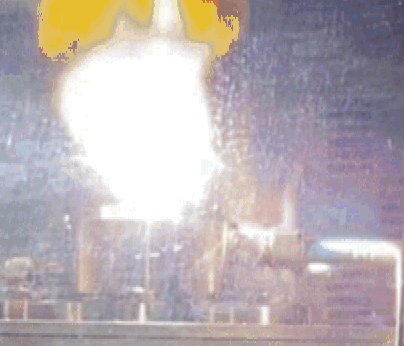 Insertion of a TPS material sample into the torch
Insertion of a TPS material sample into the torch
The UT Austin ICP torch currently operates subsonically at atmospheric pressure, but is currently being fitted with a vacuum tank to enable low-pressure and supersonic operation. The torch was developed with funding by NASA Johnson Space Center to support TPS materials testing needs of the Orion Program. The torch can be operated with test gas flows of argon, air, and mixtures of N2/O2 and Ar/CO2. The torch is stable for mixtures up to 50% CO2 in argon. The torch can run continuously at a maximum electrical input power of 50 kW, but only about half of this leads to gas heating owing to electrical dissipation and heat lost to the cooling system. It produces typical mean gas temperatures of 6000 K to 7000 K with cold-wall heat fluxes as high as 250 W/cm2.

Close up view of a material test showing yellow-hot surface (1800 K) and purple CN emission
The torch is ideal for studying gas-material interactions and ablation of candidate TPS materials. The torch has been used to study ablation of several different TPS materials including simple materials such as graphite, Fiberform and Teflon, as well as pyrolyzing ablators including polymer matrix composites (phenol and cyanate ester-based resins) and some new 3D printed and low-temperature-curing materials. The typical ablation diagnostics used for these tests includes cold-wall heat flux, surface recession rate, surface temperature (using optical pyrometry), post-test surface profiling, and material surface microstructure (optical and SEM). The hot plume and gas-surface interaction are studied using various optical diagnostic techniques including: (i) emission spectroscopy to obtain the excitation temperature, electron density, and multiple species such as N2, NO, N2+, O2, CN, OH, NH, C2, CO, N, O, C, and H, (ii) spontaneous Raman scattering to obtain time-averaged rotational and vibrational temperature of N2, O2 and CO2, (iii) laser-induced fluorescence imaging to obtain planar images of radical species such as NO, OH, CH, CN and CO, and (iv) we are developing the capability to do coherent anti-Stokes Raman spectroscopy (CARS), which we will use to make instantaneous measurements of rotational and vibrational temperature of N2, O2 and H2, and concentrations of O2 and N2.