Within the last week, more information concerning the immune mechanisms of SARS-CoV-2 has come to light. International studies characterizing the host response continue to provide updated information, and studies here in the US are beginning to gain momentum as the first wave of approval and funding reaches labs across the country. Much of what has been published concerning potential mechanisms of the viral infection have been speculations based on the previous coronaviruses (SARS-CoV-1 and MERS-CoV) and their interactions with the human immune system. With each passing week, the uniqueness of SARS-CoV-2 is being elucidated and is being used to tailor responses to the present pandemic.
Progress has been made in understanding the immunology of the infection and implementing therapies. Several follow-up convalescent plasma studies conducted in the last week have shown promise in clearing infection in patients. Details of the cellular response emerge every day and help healthcare workers and scientists redirect their efforts toward producing the most effective treatment. The next major steps include developing an understanding of how COVID-19 relates to various underlying health conditions, and how the treatment courses for those comorbidities may have to be modified to accommodate the viral infection. As we move forward, it will be more important than ever to trust the data to lead us to the best course of action.
Adaptive Immunity
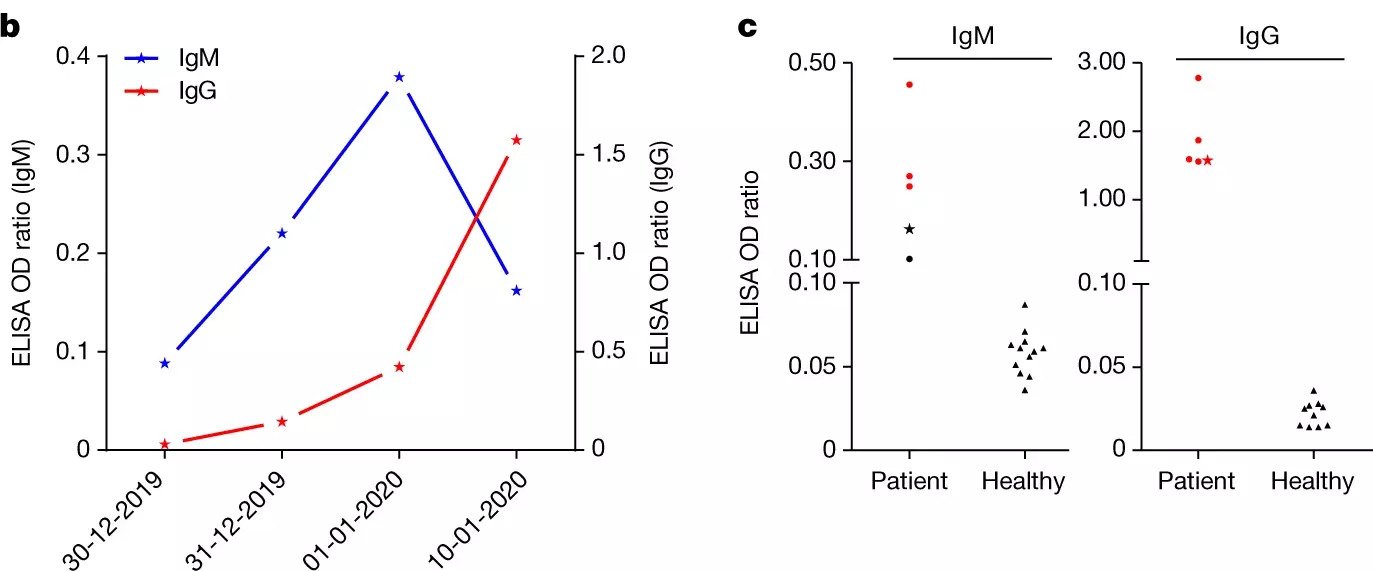
Convalescent plasma trials continue to roll out of various labs around the world, and have shown great promise in treating infected patients. Despite early hesitation by the FDA and other government agencies, plasma trials are getting underway here in the US: as of April 10, 2020, the FDA approved the first convalescent plasma trial at Houston Methodist under the direction of Drs. James Musser and Eric Salazar. Yet another plasma trial out of Wuhan, China confirmed the timeframe of the IgM and IgG peaks during the host COVID-19 response (Zhou et al., 2020). The data presented by Zhou et al. indicate that the antibody (Ab) response to SARS-CoV-2 indeed follows the pattern seen in most other pathological infections, with specific, low-affinity IgM peaking within a few days of infection and high affinity IgG peaking within the first two weeks. This timeframe highlights the critical period in the first few days post-infection in which it may be necessary to treat patients more aggressively to buy time for the adaptive immune response to mount. Similarly, the first roughly 10 days post-infection offer the most efficient collection of serum from infected patients for use in transfusional therapies, as IgG seems to peak near two weeks.
As reported last week, the primary goal of many early studies was to broadly characterize the immunological response infection as a first line in directing treatment (Zhang et al., 2020; Liu et al., 2020; Bermejo-Martin et al., 2020). Increasingly, however, specifics of the response are being elucidated. The Qin lab in China noted severe lymphopenia – a reduction in the levels of T cells, B cells, and natural killer (NK) cells – as has been described previously for both severe and non-severe infections [see April 3rd updates for details], but further reported that T cells, specifically helper T (Th) cells, seem to be more drastically affected than B or NK cells (Qin et al., 2020). Moreover, it appears that the ratio of naive Th cells to memory (mature) Th cells is increased relative to controls. This could be accounted for by the fact that the virus tends to target activated Th cells, or by the fact that it prevents naive Th cells from being activated in the first place. In either case, this prevents a fully efficacious adaptive immune response from being mounted, as Th cells are the preeminent coordinators of most immune reactions.
Diao et al. published findings that three specific cytokines (interleukin-6 (IL-6), IL-10, and tumor necrosis factor alpha (TNF-α)) are not only significantly increased in infected patients compared to controls, but are also negatively correlated with total T cell, CD8+ T cell, and CD4+ T cell counts. A pseudo-longitudinal study over the course of care for their study participants noted that as T cell levels began to increase in the recovery period, levels of the cytokines mentioned above decreased. Further, they analyzed the expression of exhaustion markers on T cells, and found PD-1 and Tim-3 to be increasingly expressed as patients progressed through the disease course (Diao, et al., 2020). Functional exhaustion is the progressive loss of function that occurs as cells perform their duties and become “worn out” over time, sometimes culminating in death of the cell (Yi, Cox, Zajac, 2010). In the context of COVID-19, this presents a potential route for supplementing the immune response by treating infected patients with anti-PD-1 or anti-Tim-3 therapies. Diao et al. hypothesize that hypersecretion of IL-6, IL-10 and TNF-α is responsible for the upregulation of exhaustion markers in response to SARS-CoV-2; immunomodulators or suppressants may be key in preventing immunological exhaustion.
Innate Immunity
The focus of innate immunological studies is to understand the cytokine storm and to tease apart its specific elements. A retrospective study of 452 patients infected with SARS-CoV-2 in Shanghai, China. demonstrated that patients with severe cases of the disease present with lymphopenia, as described previously, as well as lower percentages of myeloid cells including monocytes, eosinophils and basophils when compared with non-severe cases. Additionally, several non-cytokine biomarkers were observed, including procalcitonin and serum ferritin (Qin et al., 2020). These biomarkers represent potential indicators of disease progression, similar to the cytokines reported previously (Zhang et al., 2020; Liu et al., 2020). The exact mechanism of the cytokine storm/superantigenic component of SARS-CoV-2 remains to be determined. Understanding the details of the innate response is important for planning treatment regimens for those with underlying comorbidities, as the hyperactive immune response has been implicated as a major causal factor of the pathology of the infection.
Written by: Parker Davis
Edited by: Jina Zhou and Esther Melamed
4/13/2020
References
Abdulamir, A. S., & Hafidh, R. R. (2020). The Possible Immunological Pathways for the Variable Immunopathogenesis of COVID—19 Infections among Healthy Adults, Elderly and Children. Electronic Journal of General Medicine, 17(4), em202. https://doi.org/10.29333/ejgm/7850
Bermejo-Martin, J. F., Almansa, R., Menéndez, R., Mendez, R., Kelvin, D. J., & Torres, A. (2020). Lymphopenic community acquired pneumonia as signature of severe COVID-19 infection. Journal of Infection, 0(0). https://doi.org/10.1016/j.jinf.2020.02.029
Diao, B., Wang, C., Tan, Y., Chen, X., Liu, Y., Ning, L., Chen, L., Li, M., Liu, Y., Wang, G., Yuan, Z., Feng, Z., Wu, Y., & Chen, Y. (2020). Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19). MedRxiv, 2020.02.18.20024364. https://doi.org/10.1101/2020.02.18.20024364
Liu, H., Wang, L. L., Zhao, S. J., Kwak-Kim, J., Mor, G., & Liao, A. H. (2020). Why are pregnant women susceptible to COVID-19? An immunological viewpoint. In Journal of Reproductive Immunology (Vol. 139, p. 103122). Elsevier Ireland Ltd. https://doi.org/10.1016/j.jri.2020.103122
Qin, C., Zhou, L., Hu, Z., Zhang, S., Yang, S., Tao, Y., Xie, C., Ma, K., Shang, K., Wang, W., & Tian, D.-S. (2020). Dysregulation of Immune Response in Patients with COVID-19 in Wuhan, China. SSRN Electronic Journal. https://doi.org/10.2139/ssrn.3541136
Zhang, J. jin, Dong, X., Cao, Y. yuan, Yuan, Y. dong, Yang, Y. bin, Yan, Y. qin, Akdis, C. A., & Gao, Y. dong. (2020). Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy: European Journal of Allergy and Clinical Immunology. https://doi.org/10.1111/all.14238
Zheng, M., Gao, Y., Wang, G., Song, G., Liu, S., Sun, D., Xu, Y., & Tian, Z. (2020). Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cellular & Molecular Immunology, 1–3. https://doi.org/10.1038/s41423-020-0402-2
Zhou, P., Yang, X. Lou, Wang, X. G., Hu, B., Zhang, L., Zhang, W., Si, H. R., Zhu, Y., Li, B., Huang, C. L., Chen, H. D., Chen, J., Luo, Y., Guo, H., Jiang, R. Di, Liu, M. Q., Chen, Y., Shen, X. R., Wang, X., … Shi, Z. L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270–273. https://doi.org/10.1038/s41586-020-2012-7
Leave a Reply