April 13, 2020 by Sam Bazzi
In a review published by Neurology this week, recommendations were made on how to adjust disease-modifying therapies (DMTs) for people with MS who are infected with SARS-CoV-2 (Brownlee, et al., 2020). In summary, MS patients with minor COVID-19 symptoms should not alter their therapy, as the risk of MS symptoms worsening is generally not worth the potential benefit of altering therapy. If symptoms of COVID-19 are severe, the authors recommend that physicians should consider altering DMTs “with greater immunosuppressant effects. “
It is currently unknown if MS patients are at a higher risk of COVID-19 infection, or if they are at risk of developing more severe symptoms if they get infected. In general, people with MS are at higher risk of infection with pneumonia and influenza (especially if they have bulbar weakness), but generally not upper respiratory tract infections (Wijnands JM et al., 2017, Marrie RA et al., 2014). In addition, patients are at a higher risk of ICU admission for infections and have a higher 1-year mortality rate after admission. Therefore, those with autoimmune conditions such as MS or NMOSD should be particularly careful about adhering to the infection prevention guidelines set out by the CDC and WHO.
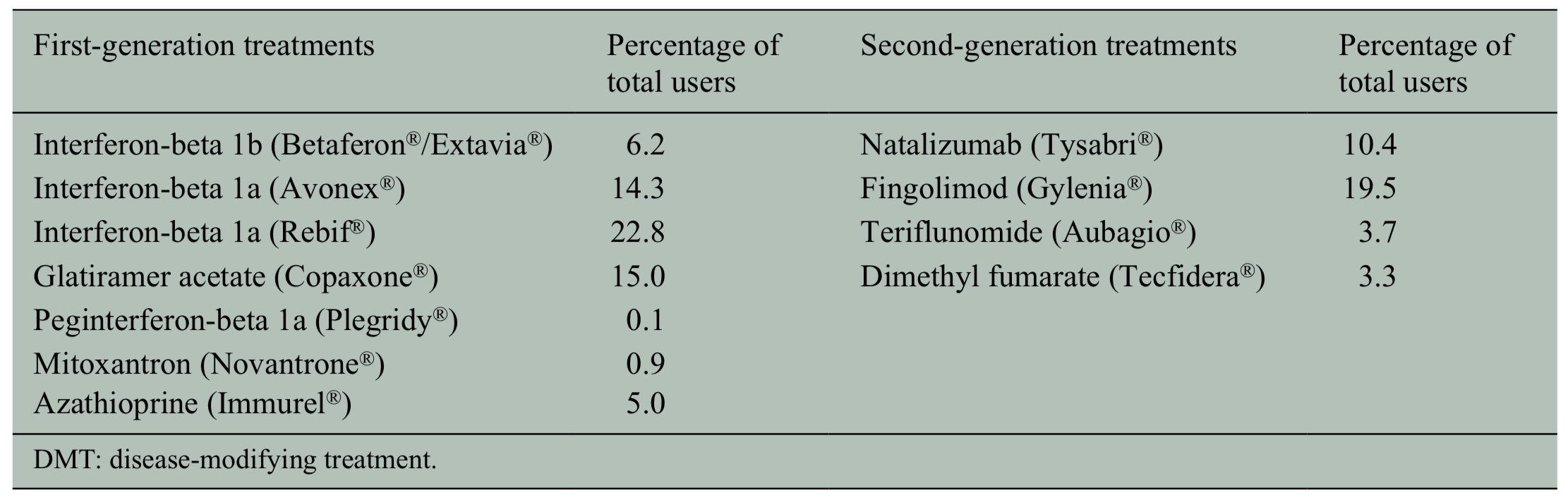
Another important consideration is how particular DMTs affect infection risk. A 2018 paper in the Journal of Neurology, Neurosurgery, and Psychiatry found that first generation DMTs were not associated with higher infection risk, but second generation DMTs were (see Table 1 below). This effect with second generation DMTs was largely driven by natalizumab (Tysabri). Natalizumab was associated with a 59% higher chance of infection risk (Wijnands, et al., 2018). Alemtuzumab and cladribine lead to a period of lymphopenia (low level of lymphocytes in the blood), which could increase the chance of infection and should be considered during the time of COVID-19.
During COVID19, extended interval dosing of DMTs has been suggested for consideration in patients on anti-CD20 B-cell depleting therapies, including ocrelizumab and rituximab, especially for patients who continue to be B-cell depleted beyond 6 months since last medication dose (Brownlee, et al., 2020.) Extended interval dosing strategy is already used for natalizumab in context of decreasing the risk of developing progressive multifocal leukoencephalopathy. Another consideration is whether or not to use steroids during COVID-19. While chronic use of steroids may predispose to higher risk of infection, short term use of steroids may be beneficial in MS patients with worsening of MS symptoms due to COVID-19 (Brownlee, et al., 2020).
Another interesting point to consider is the importance of fever management for MS patients with COVID-19. Fevers can temporarily worsen existing symptoms for people with MS (due to Uhthoff phenomenon) and even bring back old symptoms that had resolved (Brownlee, et al., 2020). Thus, MS patients should be counseled about fever management if they develop COVID-19.
Written by: Sam Bazzi
Edited by: Jina Zhou and Esther Melamed
4/13/2020
References
Battaglia, M., Kobelt, G., Ponzio, M., Berg, J., Capsa, D., Dalén, J., & European Multiple Sclerosis Platform. (2017). New insights into the burden and costs of multiple sclerosis in Europe: Results for Italy. Multiple Sclerosis Journal, 23(2_suppl), 104-116.
Brownlee, W., Bourdette, D., Broadley, S., Killestein, J., & Ciccarelli, O. (2020). Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. Neurology.
Wijnands, J. M. A., Zhu, F., Kingwell, E., Fisk, J. D., Evans, C., Marrie, R. A., … & Tremlett, H. (2018). Disease-modifying drugs for multiple sclerosis and infection risk: a cohort study. Journal of Neurology, Neurosurgery & Psychiatry, 89 (10), 1050-1056.
Leave a Reply