Several surprising announcements were made this past week pertaining to COVID-19 clinical trials. The first was the ending of the first phase of the NIAID ACTT trial of remdesivir. Although remdesivir did reduce recovery time from COVID-19 it did not demonstrate a significantly better mortality rate; thus, NIAID has begun the next phase of ACTT, respectively called ACTT2, which is a joint trial of remdesivir and baricinib, an anti-inflammatory inhibitor of JAK1 and JAK2.
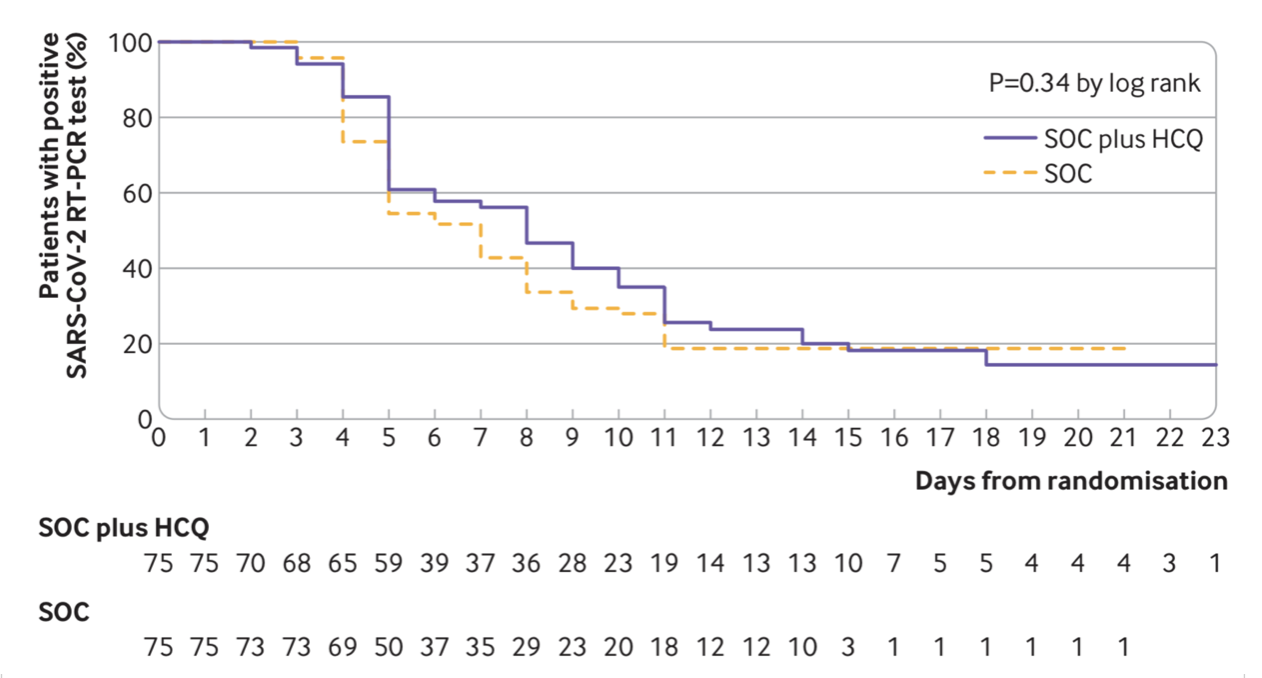
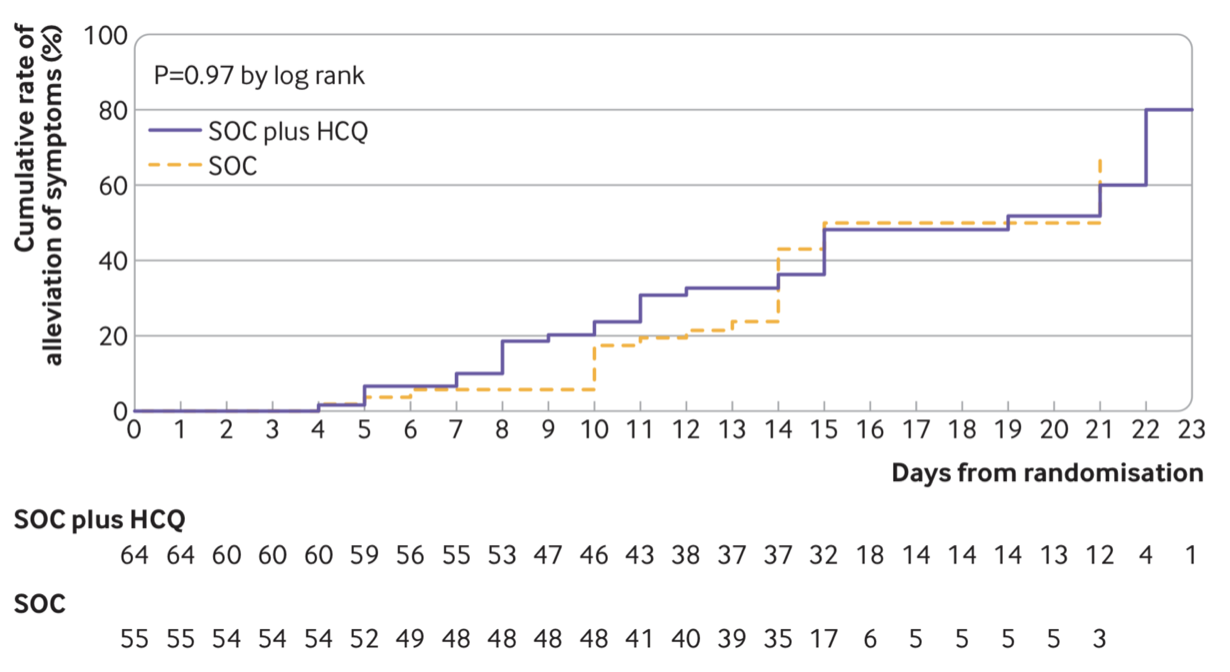
This week another study has also been released evaluating hydroxychloroquine in COVID-19 mild and moderate cases (Tang W. et al. 2020). Once again, there was no reported benefit of the therapy as shown in Figure 1 and 2. Also interestingly, the NIH/NIAID has announced another clinical trial of hydroxychloroquine and azithromycin despite the limited evidence for their efficacy so far (check last week’s blog post on this page!). However, this trial is a preventative trial in which the therapy is being used preemptively to ward off SARS-CoV-2 infection. In this double blind randomized clinical trial, a total of 2000 patients will be recruited and randomly assigned to hydroxychloroquine with azithromycin or the placebo group. Patients will be monitored for COVID-19 symptoms as well as side effects from hydroxychloroquine with azithromycin. The rate and severity of infection will be compared between the groups to see if hydroxychloroquine still has a place in COVID-19 therapy arena.
Finally, some preliminary data from the triple therapy trial of interferon beta-1b, lopinavir-ritonavir, and ribavirin has been published (Ngai Hung et al. 2020). The triple therapy reportedly reduced time to RT-PCR negative test status. However, no health related primary outcome, such as time to alleviation of symptoms or reduced mortality, was reported. While time to negative status is a useful metric, the key factor in progressing to an FDA approved treatment for COVID-19 is a therapy that reduces both mortality and time to symptom alleviation. Thus, clinical trials continue to strive forward to find a drug that addresses both issues!
References
Tang W, et al. (2020) Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ369:m1849.
Hung IF-N, et al. (2020) Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. doi:10.1016/S0140-6736(20)31042-4.
Leave a Reply