Following the preliminary results from COVID-19 trials in the previous weeks, ongoing trials are continuing to be revamped and redesigned. So while we wait for additional news, this week’s blog post is going to focus on the debate of corticosteroids as a COVID-19 treatment.
Corticosteroids are used for a variety of conditions such as asthma, rheumatological and neurological conditions, and allergies. Steroids are known to have anti-inflammatory, immunosuppressive, and vasoconstrictive effects. At this time however, there is conflicting information on the success of steroid treatment in COVID-19.
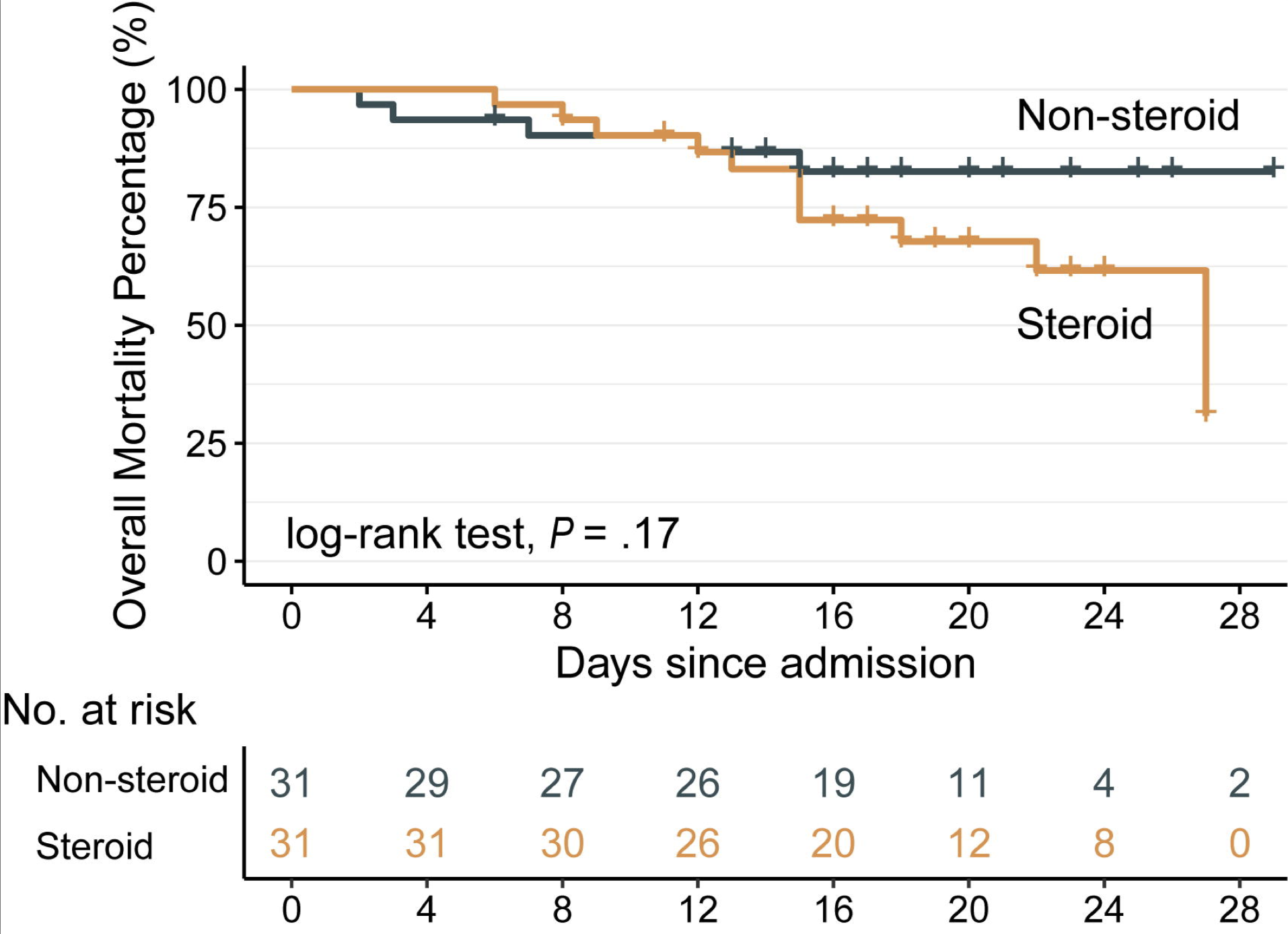
A large majority of clinical trials at this point present detrimental effects from the long-term use of corticosteroids in COVID-19. However, the exact nature of this effect varies greatly between reports. The results from three of the initial studies in China ranged from no significant difference, increased risk of ICU admission, and longer time to RT-PCR negative testing status (Liu et al. 2020, Wang et al. 2020, Ling et al. 2020, and Veronese N, et al. 2020). Since these initial studies, several other studies have re-emphasized these findings and found a non-significant trend of steroid use leading towards lower survival probability as shown in Figure 1 (n=151, Lu et al. 2020). Collectively, these results have led the World Health Organization to recommend against the use of corticosteroids for COVID-19 and the CDC to report prolonged use of corticosteroids as a risk factor for COVID-19.
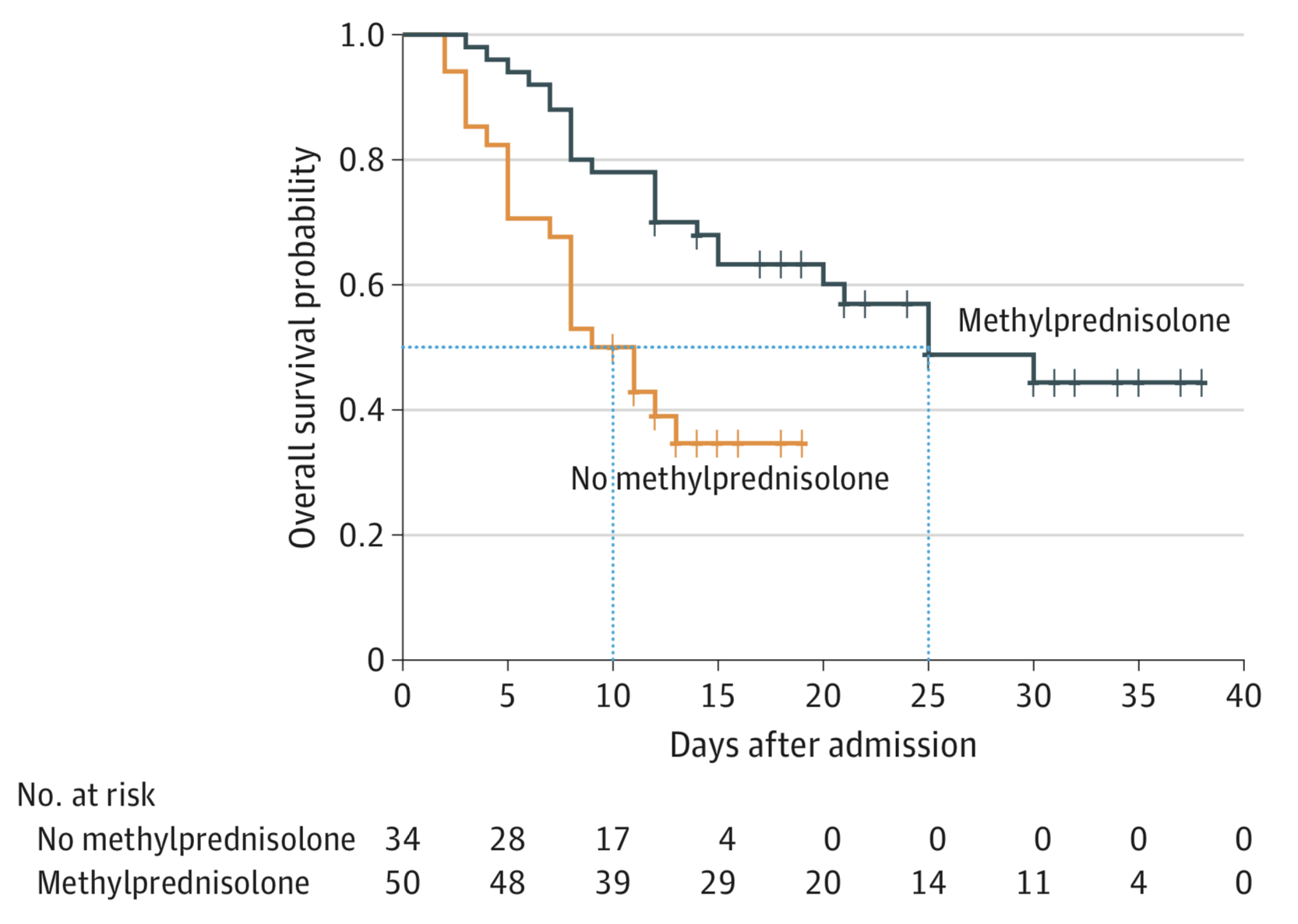
However, other reports have found steroids reduced the risk of death in patients with ARDS and reduced escalation of care including movement from the wards to the ICU, mechanical ventilation, or mortality (Wu et al. 2020 and Fadel et al. 2020). The results presented by Wu et al. 2020, have led toward a wider use of corticosteroids in China. However, the reported reduction of mortality in COVID patients with ARDS was only reduced to 46.0% from 61.8% with a small sample size of n=50 for the corticosteroids (methylprednisolone) and n=30 for the control standard of care (SOC). However, Fadel et al. 2020 tested a short dosing of corticosteroids early on in COVID in their clinical trial, with this timing and duration possibly contributing as a key factor in their reported success. Although the study had a relatively small sample size (n=81 for SOC and n=132 for corticosteroids) and compound therapy of corticosteroids plus hydroxychloroquine, the positive results suggest shorter treatments of steroids may be beneficial with larger follow up studies needed.
As shown in Figure 1 and 2, there is a stark contrast in the effects of corticosteroids in COVID-19 in the literature, and there is scant data thus far to validate the use of steroids or their discontinuation during COVID-19 (Lu et al. 2020 and Wu et al. 2020). It is clear from this debate, though, that ongoing clinical trials to evaluate the role of corticosteroids in COVID-19 will be critical toward ascertaining the relationship of corticosteroids to COVID-19 risk and disease progression, and will help guide the use of steroids for other conditions during the pandemic.
References
Kui L, et al. (2020) Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl). doi:10.1097/CM9.0000000000000744.
Wang D, et al. (2020) Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA – J Am Med Assoc. doi:10.1001/jama.2020.1585.
Ling Y, et al. (2020) Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl). doi:10.1097/CM9.0000000000000774.
Veronese N, et al. (2020) Use of Corticosteroids in Coronavirus Disease 2019 Pneumonia: A Systematic Review of the Literature. Front Med 7:170.
Lu X, et al. (2020) Adjuvant corticosteroid therapy for critically ill patients with COVID-19. medRxiv. doi:10.1101/2020.04.07.20056390.
Wu C, et al. (2020) Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. doi:10.1001/jamainternmed.2020.0994.
Fadel R, Morrison AR, et al. COVID-19 Management Task Force, Early Short Course Corticosteroids in Hospitalized Patients with COVID-19, Clinical Infectious Diseases, https://doi.org/10.1093/cid/ciaa601
Leave a Reply