In the process of lymphocyte development, progenitor T and B cells undergo several rounds of signaling and selection before being released to the periphery to fight infections. As part of the normal developmental routine, immature lymphocytes undergo “negative selection” in which cells bearing autoreactive (self-reactive) antigen receptors are removed from the population, thereby preventing potential future reactions against the host’s own body (Palmer, 2003; Melchers et al., 1995).
Viral infections often cause major damage to host cells and tissues, setting the stage for serious and even life-threatening secondary sequelae. In particular, autoimmune conditions can develop as a result of viral infections via a number of mechanisms: a dysregulated inflammatory response can continue damaging the host even after the pathogen has been cleared; infection of “immunologically privileged” sites – tissues generally sequestered from the central and peripheral immune systems like the brain, eyes, germ cells, etc. – can cause the release from these tissues of antigens to which the peripheral immune system was previously ignorant, leading to an autoimmune response; and viral proteins can even retain enough similarity to host proteins to elicit the formation of antibodies that, consequently, are also autoreactive. These mechanisms present a “workaround” for the development of autoimmunity despite the successful (though usually incomplete) purging of autoreactive lymphocytes in normal development.
Immune thrombocytopenic purpura (ITP) is one such autoimmune condition that has been established as a sequela of pathogenic infection. Specifically, its development and progression has been observed post-infection with the human immunodeficiency virus (HIV)-1, hepatitis C virus (HCV), the bacteria Helicobacter pylori, and even SARS-CoV-1 (Liebman, 2008; Cines, Blanchette, 2002). ITP is characterized by a low platelet count (thrombocytopenia) and mucocutaneous bleeding and comes in two major forms, primary (idiopathic) and secondary, which have slightly different presentations and courses of progression. In primary ITP, autoreactive IgG attacks the body’s own platelets and opsonizes them for clearance in the spleen and liver – that is, the IgG molecules coat the surface of the platelets and mark them for destruction by innate leukocytes like macrophages (Cines, Blanchette, 2002). Secondary ITP is just that: it occurs secondarily to some other underlying condition, whether that is a lymphoproliferative disorder like chronic lymphoblastic leukemia or Hodgkin’s lymphoma, or an infection. Secondary ITP is characterized by accelerated platelet destruction and/or impaired platelet production, though the source of this pathology can vary from patient to patient. Treatment of secondary ITP generally focuses on targeting the underlying condition rather than on restoring platelet counts directly (Liebman, Stasi, 2007; Liebman, 2009).
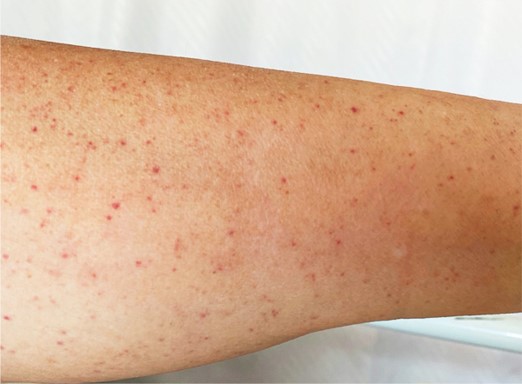
Recently, ITP has been diagnosed in several COVID-19 patients. Zulfiqar et al. reported a case of a 65-year-old woman who presented to the emergency department with hypertension and autoimmune hypothyroidism, fatigue, fever, dry cough, and abdominal discomfort. Upon examination, she had a normal white blood cell count, normal hemoglobin, and normal platelet counts, but a slightly elevated C-reactive protein (CRP) level, indicative of an ongoing inflammatory response. Indeed, she subsequently tested positive for COVID-19 via oropharyngeal swab. On day 4 post-admission, the patient developed purpura on her lower extremities (Figure 1) and had a platelet count of 66,000 platelets/mm3, which dropped to 8,000/mm3 by day 7 (normal platelet counts are between ~150,000/mm3 and ~450,000/mm3). Two doses of intravenous immunoglobulin (IVIg) had little effect on the patient’s thrombocytopenia; a platelet transfusion on day 9 combined with eltrombopag (75 mg/day) led to recovered platelet counts (139,000/mm3) by day 13, at which time the purpura also disappeared (Zulfiqar et al., 2020).
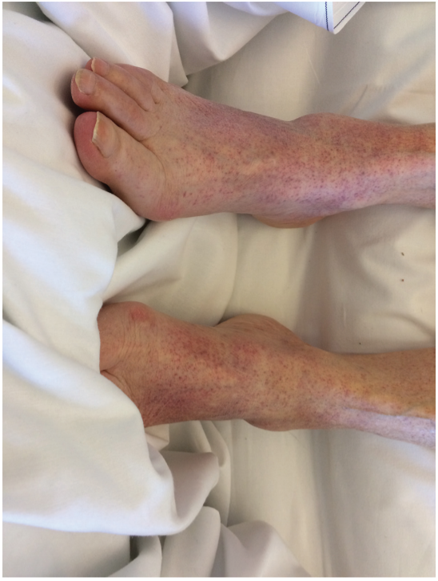
Another case of ITP following SARS-CoV-2 infection was reported recently in the Journal of Medical Cases. An 86-year-old female presented to the hospital with dyspnea (trouble breathing) and a cough, as well as petechial lesions on her limbs, lower back, and oral mucosa that had appeared 24 hours prior to her admission (Figure 2). No abnormal leukocytosis was observed, though the patient did have a moderate CRP elevation, again indicative of an inflammatory response. Her complete blood count (CBC) demonstrated severe thrombocytopenia (7000/mm3), and she tested PCR-positive for SARS-CoV-2 infection. Platelet transfusions occurred on day 1 and day 2 post-admission, though without significant initial effect. Corticosteroid treatment, often used to ameliorate severe inflammation, was started on day 2 in addition to IVIg, and by day 7 the platelet count had risen to 144,000/mm3. The patient was discharged two weeks later with a stable platelet count (Gonze et al., 2020).
Thrombocytopenia is a condition that was frequent as a result of SARS-CoV-1 infection, but ITP, though associated with other viruses, is relatively rare in association with coronaviruses. In the original SARS coronavirus outbreak, ITP was found to be triggered by various antibiotics and antivirals, heparin, hemodialysis, and even extracorporeal membrane oxygenation (ECMO). Though currently unknown, the mechanisms of thrombocytopenia, both immune and non-immune, in COVID-19 are hypothesized to be similar to the mechanisms observed in SARS-CoV-1 infection: decreased platelet production caused by the cytokine storm, and infection of bone marrow stromal cells and hematopoietic stem cells; increased platelet destruction as a result of autoreactive antibodies and the formation of immune complexes; and a decreased circulating platelet count due to lung injury (Yang et al., 2020). The fact that steroids were effective in restoring platelet counts in these few case studies may suggest the significant role played by the inflammatory cytokine storm in inducing thrombocytopenia, as these steroids would have helped moderate the effects of the cytokine release. Again, ITP is relatively rare in SARS-CoV-2 infection, but acts as a marker of potential “side effects” of infection and presents a new angle to take into consideration when determining the best course of treatment for a given patient.
Written by: Parker Davis
Edited by: Jina Zhou and Esther Melamed
6/8/2020
References
Cines, D. B., & Blanchette, V. S. (2002). Medical progress: Immune thrombocytopenic purpura. In New England Journal of Medicine (Vol. 346, Issue 13, pp. 995–1008). Massachusetts Medical Society. https://doi.org/10.1056/NEJMra010501
Gonze, A., Gonze, A., Hannesse, C., Coppens, N., Vellemans, H., & Maury, G. (2020). SARS-CoV-2-Induced Severe Immune Thrombocytopenic Purpura. Journal of Medical Cases, 11(6), 166–168. https://doi.org/10.14740/jmc.v11i6.3481
Liebman, H. A. (2008). Viral-associated immune thrombocytopenic purpura. Hematology / the Education Program of the American Society of Hematology. American Society of Hematology. Education Program, 2008(1), 212–218. https://doi.org/10.1182/asheducation-2008.1.212
Liebman, H. A. (2009). Recognizing and Treating Secondary Immune Thrombocytopenic Purpura Associated With Lymphoproliferative Disorders. Seminars in Hematology, 46(SUPPL. 2). https://doi.org/10.1053/j.seminhematol.2008.12.004
Liebman, H. A., & Stasi, R. (2007). Secondary immune thrombocytopenic purpura. In Current Opinion in Hematology (Vol. 14, Issue 5, pp. 557–573). https://doi.org/10.1097/MOH.0b013e3282ab9904
Melchers, F., Rolink, A., Grawunder, U., Winkler, T. H., Karasuyama, H., Ghia, P., & Andersson, J. (1995). Positive and negative selection events during B lymphopoiesis. Current Opinion in Immunology, 7(2), 214–227. https://doi.org/10.1016/0952-7915(95)80006-9
Palmer, E. (2003). Negative selection – Clearing out the bad apples from the T-cell repertoire. In Nature Reviews Immunology (Vol. 3, Issue 5, pp. 383–391). Nature Publishing Group. https://doi.org/10.1038/nri1085\
Yang, Y., Zhao, J., Wu, J., Teng, Y., & Xia, X. (2020). A Rare Case of Immune Thrombocytopenic Purpura Secondary to COVID‐19. Journal of Medical Virology, jmv.26051. https://doi.org/10.1002/jmv.26051
Zulfiqar, A. A., Lorenzo-Villalba, N., Hassler, P., & Andrès, E. (2020). Immune Thrombocytopenic Purpura in a Patient with COVID-19. In New England Journal of Medicine (Vol. 382, Issue 18, p. E43). Massachussetts Medical Society. https://doi.org/10.1056/NEJMc2010472
Leave a Reply