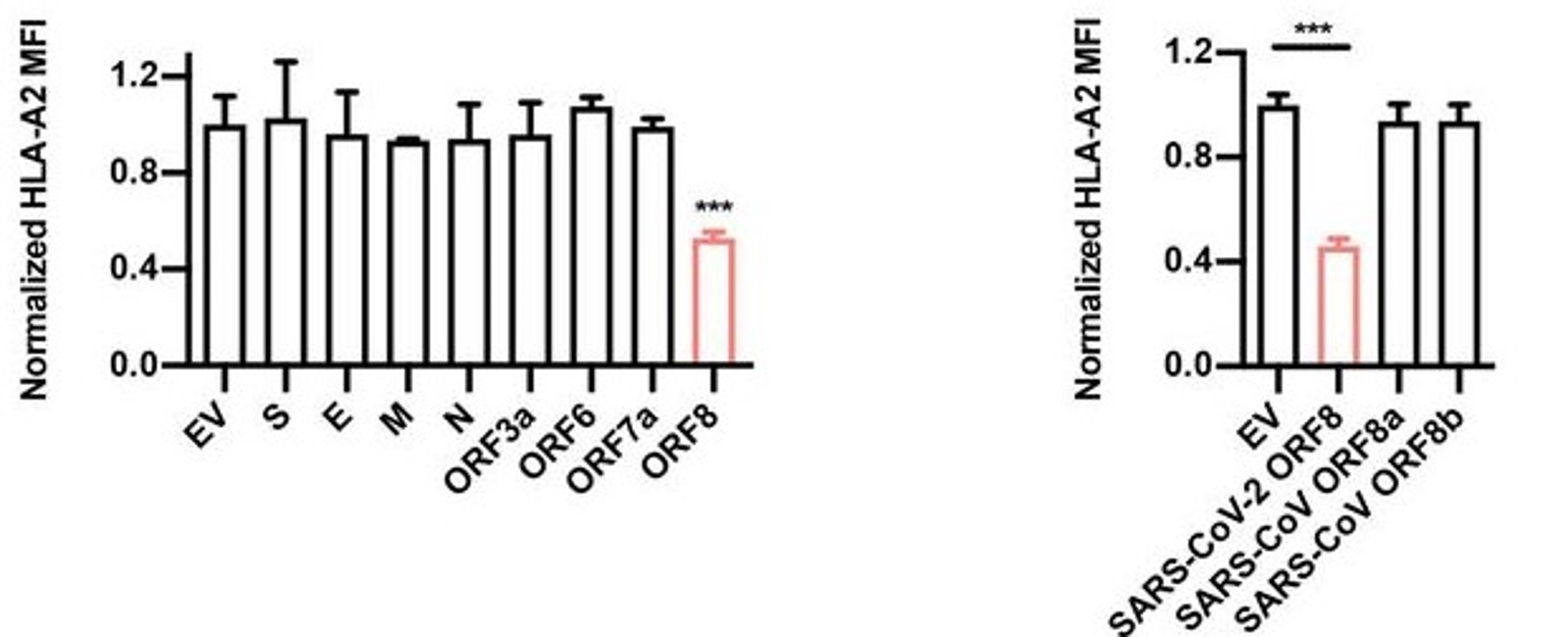
Though much progress has been made in determining the specific interactions between SARS-CoV-2 infectious particles and host cells (Liang et al., 2020; see updates for week of 6/2/2020), we are only beginning to understand the exact mechanisms of pathogenicity in detail. As part of this ongoing inquiry, Zhang et al. surveyed the four structural proteins of SARS-CoV-2 (spike [S], envelope [E], membrane [M], and nucleocapsid [N]) and several accessory proteins found throughout the virus’ genome to assess what effects they have on pathogenicity. The authors found that one accessory protein, encoded by open reading frame (ORF) 8, was able to significantly inhibit a host cell’s ability to initiate an immune response by downregulating expression of the Major Histocompatibility Complex (MHC) I protein by these cells (Figure 1).
Briefly, the MHC is a family of molecules that comes in two major classes (I and II), each composed of multiple subclasses and allotypes that give rise to a diverse array of functional proteins. Both MHC I and II are involved with the presentation of antigens and activation of immune responses, but they act at separate, yet interdependent, stages. MHC I is a molecule that is expressed on almost all of a host organism’s cells (non-nucleated cells like red blood cells do not express MHC I), and allows presentation of antigens to innate immune cells – typically dendritic cells – that then relay the signal to T cells in lymph nodes and the spleen (York, Rock, 1996). The presence of functional MHC I on the surface of host cells is required for proper development of CD8+ T cells, and therefore for the development of a potent cell-mediated immune response. Any manner of interference that alters the components of the molecule or its associated protein complexes (such as tapasin, the gatekeepers TAP1 and TAP2, or an enzyme called ERp57, among the proteins that make up the peptide loading complex of MHC I; Blees et al., 2017) will inhibit proper functioning. Similarly, mutations or interactions with pathogenic molecules may prevent MHC I from binding an antigen within the cell and being trafficked to the surface, thereby acting to suppress the immune response of the organism and diminish its capacity to clear an infection. This interference may manifest in the form of random mutations, or via interactions with non-host molecules, as in the case of viral infection.
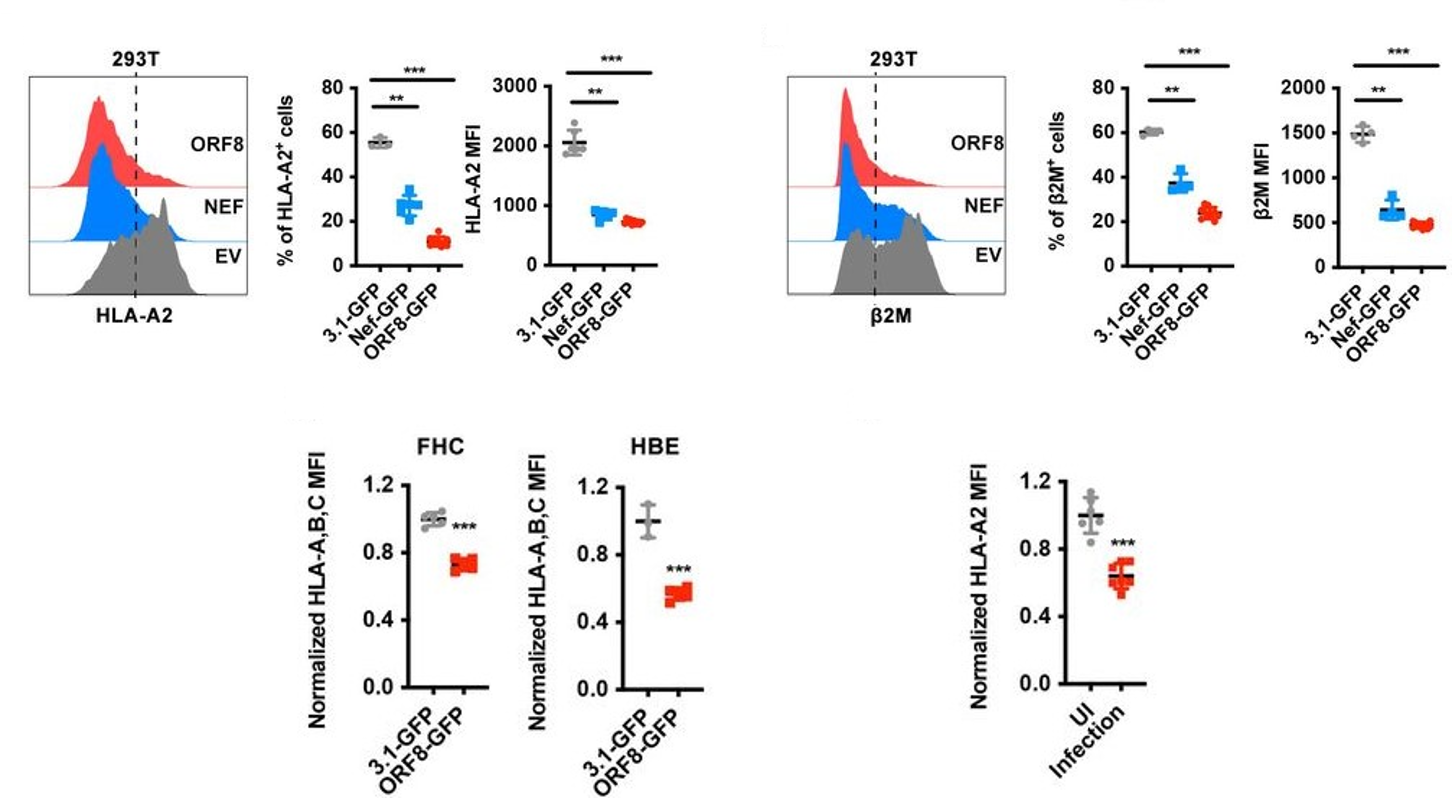
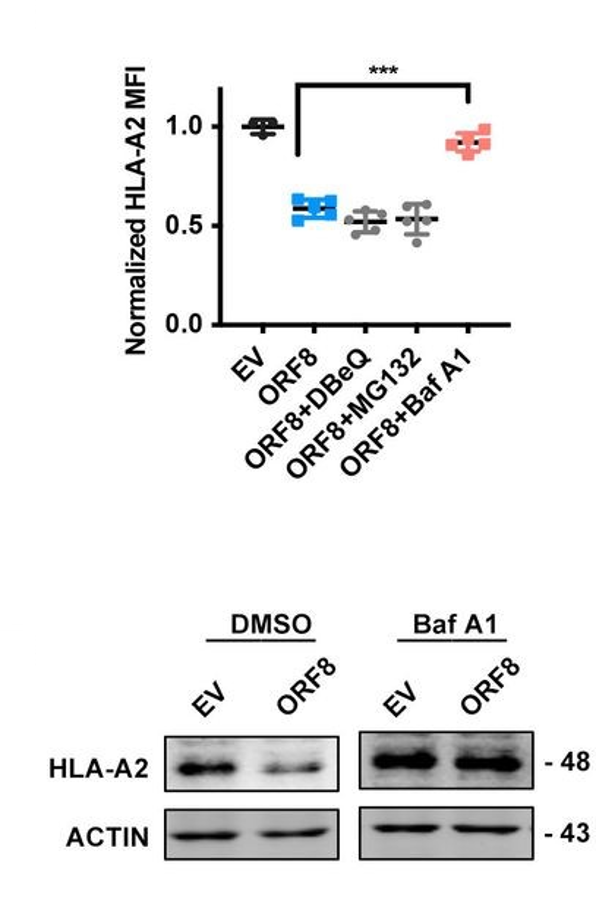
By transfecting HEK293T cells with ORF8-expressing or control-expressing plasmids, the authors of this study were able to determine in great detail the mechanism of the observed downregulation of MHC I. Using flow cytometry, they found that both the frequency and the mean fluorescence intensity of the heavy chain and beta-2-microglobulin components of the intact MHC I protein were significantly reduced in the ORF8-expressing cells relative to the control cells (Figure 2, top row), and that this reduction occurred in a dose-dependent manner. They then repeated the same plasmid experiment using other cell lines (human fetal colon cells, human bronchial epithelial cells, and human liver cells) and, in a separate experiment, infected HEK293T with authentic SARS-CoV-2 viral particles, all of which yielded results similar to the original trial – in other words, MHC I was downregulated regardless of cell line or origin of the expressed ORF8 protein (Figure 2, bottom row). When HEK cells were treated with various inhibitors of protein degradation, only bafilomycin A1, an inhibitor of lysosomal degradation, significantly counteracted the effects of ORF8 (Figure 3). The other inhibitors tested (N2, N4-dibenzylquinazoline-2,4-diamine [DBeQ]; and MG132) block endoplasmic reticulum-associated protein degradation and ubiquitin-protease-associated degradation, respectively. These results suggest a role for ORF8 in the targeting of MHC I complex molecules to lysosomes for degradation. Such sequestration ensures that the MHC molecules cannot bind antigen within endosomal compartments and never reach the cell surface for presentation of this antigen. This interaction is immunosuppressive for the reasons outlined above.
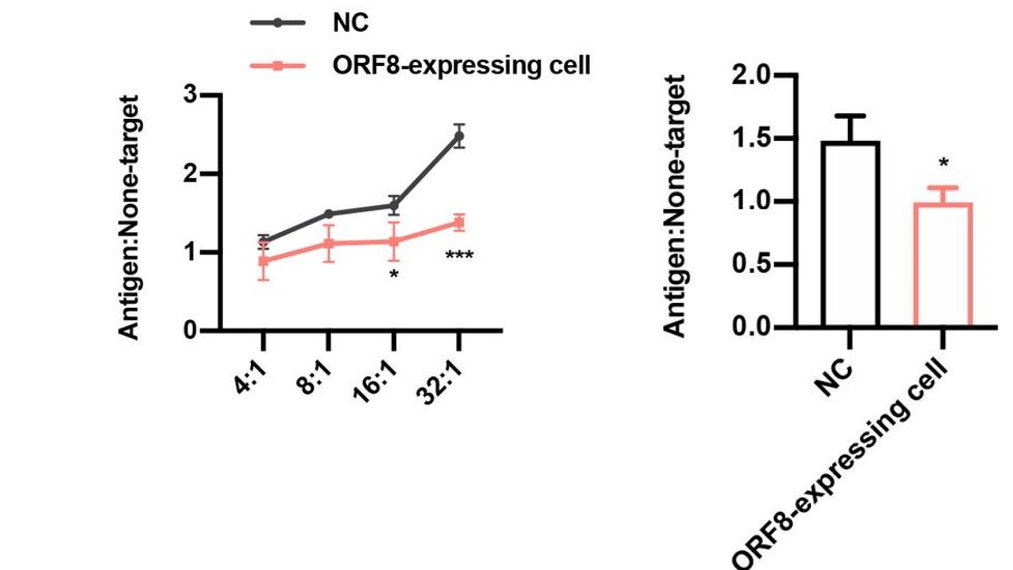
SARS-CoV spike protein-derived peptide-1 (SSp-1) is an epitope (the specific region of an antigen on the scale of molecules or even atoms that is recognized by immune cells) present in the spike protein of both SARS-CoV and SARS-CoV-2. The overall sequence homology of the spike protein between these two viruses is relatively low. However, this specific epitope represents an island of similarity that is hypothesized as a potential target through which the host immune system may recognize SARS-CoV-2, given its involvement in the recognition of SARS-CoV (Grifoni et al., 2020). When SSp-1-specific cytotoxic lymphocytes (CTLs) were directed against ORF8-expressing and control HEK293T cells, the lymphocytes were only able to eliminate the ORF8-expressing cells with a much lower efficiency than the control cells (Figure 4, left). And when CD8+T cells from recovered COVID-19 patients were directed against the same sets of HEK cells, they too could only eliminate the ORF8-expressing cells with an impaired efficiency relative to the control cells (Figure 4, right). These results reflect the inability of the HEK cells to successfully express antigen on their surface, due to a lack of functional MHC I protein.
In all, this paper marks an important step toward understanding the exact mechanisms of SARS-CoV-2 pathogenicity. Developing an understanding of specific interactions between SARS-CoV-2 molecules and host molecules (for example, the interaction between viral non-structural proteins 9/10 and host nuclear factor-kappa B-repressing protein [Liang et al., 2020], or between ORF8 and MHC I outlined here) will move us further in the direction of producing therapies that are incredibly potent and, importantly, virus specific. The authors note that their findings suggest a mechanism for autophagy mediated by the endoplasmic reticulum that is responsible for the transference of MHC I to lysosomes. The elucidation of this mechanism, if it indeed exists, should be a priority for the continuation of the work outlined here. Future studies should focus on understanding the specifics of these processes and on developing therapies that exploit faults in these interactions to fight infection.
Written by: Parker Davis
Edited by: Jina Zhou and Esther Melamed
6/15/2020
References
Blees, A., Januliene, D., Hofmann, T., Koller, N., Schmidt, C., Trowitzsch, S., Moeller, A., & Tampé, R. (2017). Structure of the human MHC-I peptide-loading complex. Nature, 551(7681), 525–528. https://doi.org/10.1038/nature24627
Grifoni, A., Sidney, J., Zhang, Y., Scheuermann, R. H., Peters, B., & Sette, A. (2020). Candidate Targets for Immune Responses to 2019-Novel Coronavirus (nCoV): Sequence Homology- and Bioinformatic-Based Predictions. SSRN Electronic Journal. https://doi.org/10.2139/ssrn.3541361
Thieme, C. J., Anft, M., Paniskaki, K., Blazquez-Navarro, A., Doevelaar, A., Seibert, F. S., Hoelzer, B., Konik, M. J., Brenner, T., Tempfer, C., Watzl, C., Dolff, S., Dittmer, U., Westhoff, T. H., Witzke, O., Stervbo, U., Roch, T., & Babel, N. (2020). The SARS-CoV-2 T-cell immunity is directed against the spike, membrane, and nucleocapsid protein and associated with COVID 19 severity. MedRxiv, 2020.05.13.20100636. https://doi.org/10.1101/2020.05.13.20100636
York, I. A., & Rock, K. L. (1996). Antigen Processing and Presentation by the Class I Major Histocompatibility Complex. Annual Review of Immunology, 14(1), 369–396. https://doi.org/10.1146/annurev.immunol.14.1.369
Zhang, Y., Zhang, J., Chen, Y., Luo, B., Yuan, Y., Huang, F., Yang, T., Yu, F., Liu, J., Liu, B., Song, Z., Chen, J., Pan, T., Zhang, X., Li, Y., Li, R., Huang, W., Xiao, F., & Zhang, H. (2020). The ORF8 Protein of SARS-CoV-2 Mediates Immune Evasion through Potently Downregulating MHC-I. BioRxiv. https://doi.org/10.1146/annurev.immunol.14.1.369
Leave a Reply