The RECOVERY trial out of the United Kingdom announced their preliminary results on hydroxychloroquine efficacy for COVID-19. Using the primary endpoint of mortality at day 28 post symptoms onset, they enrolled a total of 1542 patients randomised to hydroxychloroquine and 3132 patients randomised to standard care. Overall, they found there was no significant difference between the groups (25.7% hydroxychloroquine vs. 23.5% usual care; hazard ratio 1.11 [95% confidence interval 0.98-1.26]; p=0.10), and “have concluded that there is no beneficial effect of hydroxychloroquine in patients hospitalised with COVID-19” (RECOVERY Trial).
Despite the lack of proven efficacy, hydroxychloroquine still has many ongoing trials with several focusing on using the drug preemptively to reduce risk of infection. While these trials started later in the COVID-19 response, the first results of this preemptive use have been published this week in the Lancet (Boulware DR, et al. 2020). Boulware et al. found that hydroxychloroquine did not reduce the rate of infection in individuals working closely with patients having confirmed COVID-19 cases (including the populations of family members and healthcare workers), as shown in Figure 1. This trial does have some limitations such as the short time frame studied of 14 days, so it will be interesting to see larger trial results on this topic evaluating hydroxychloroquine at later timepoints beyond day 14.
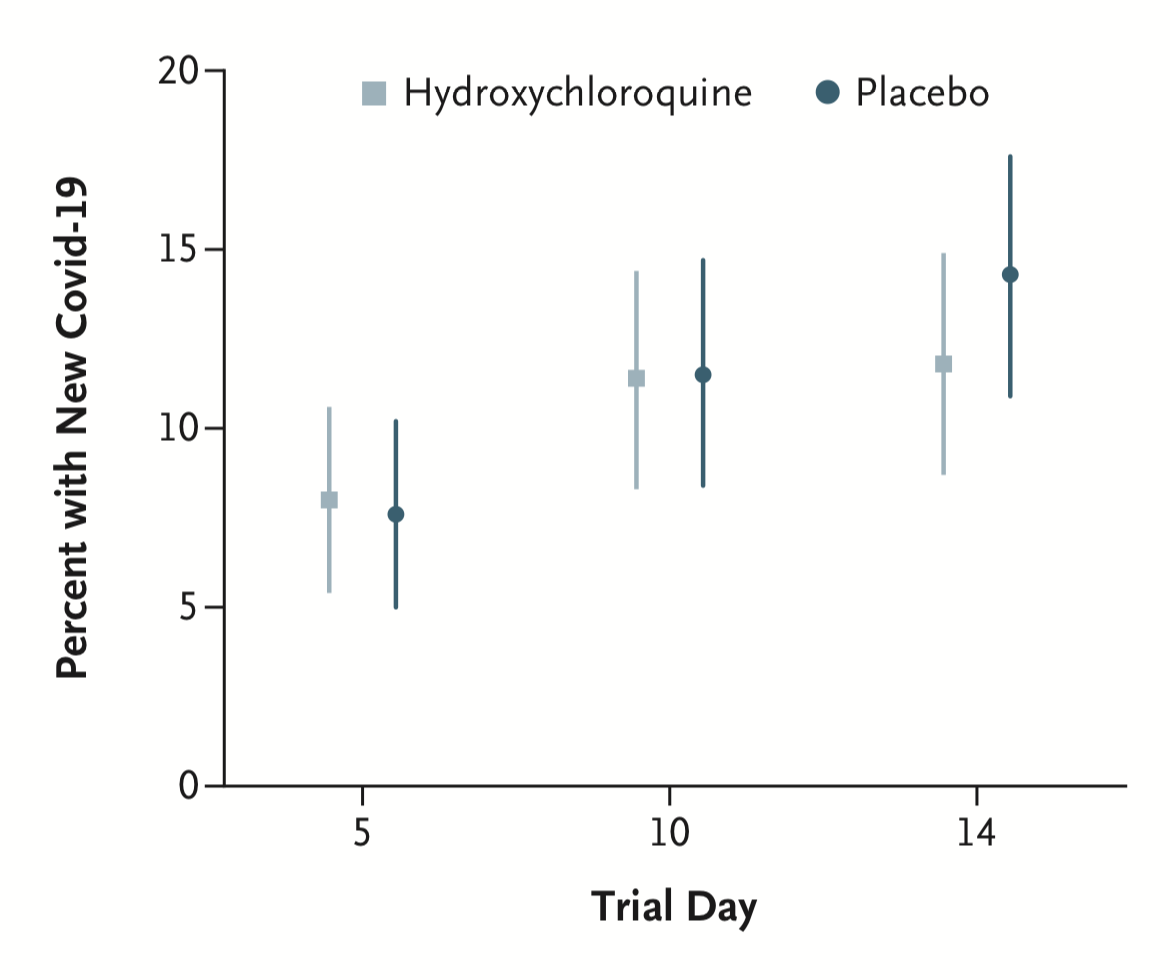
This week, there was also an announcement of the second SARS-CoV-2 antibody clinical trial. This trial from Regeneron Pharmaceuticals is for an antibody cocktail mixture with both human and humanized murine antibodies. The trial will begin in mid-June and recruit or evaluate both hospitalized and non-hospitalized patients in addition to testing the therapy as a preventative treatment in high risk groups. It will be interesting to see how the antibody cocktail compares to the single antibody therapies for COVID-19 in the future. Regeneron also announced two publications have been accepted by Science on the results of the antibody cocktail, but at this time the papers are unavailable.
The Moderna mRNA vaccine of the spike protein has garnered a large amount of attention in the media and is one of the vaccines furthest along in clinical trials. Moderna confirmed that a Phase III trial will begin in July using a 100 ug single dose model of the mRNA to avoid potential complications observed in a small fraction of patients at the higher 250 ug dose. A total of 30,000 patients will be enrolled and split between the vaccinated and placebo groups.
The NIH has also announced a new clinical study that will focus specifically on therapies being used in children with COVID-19. Many clinical trials do not look at this age group due to the ethical and logistical challenges behind clinical trials in children. However, physicians often have to treat children with drugs that have not been well studied in the age group. The NIH is hoping to monitor the pharmacokinetics in such patients during COVID-19 to better understand how these drugs are metabolized and affect children.
References
Boulware DR, et al. (2020) A Randomized Trial of Hydroxychloroquine as Postexposure Prophylaxis for Covid-19. N Engl J Med. doi:10.1056/NEJMoa2016638.
Regeneron Pharmaceuticals: https://www.regeneron.com/covid19
Moderna Press Release on Phase III Trial: https://investors.modernatx.com/news-releases/news-release-details/moderna-advances-late-stage-development-its-vaccine-mrna-1273
NIH Children Drug Evaluation Clinical Trial: https://www.nih.gov/news-events/news-releases/nih-funded-study-evaluate-drugs-prescribed-children-covid-19
Leave a Reply