Though ACE2 has been identified as the primary mediator of SARS-CoV-2 attachment and entry into host cells, various other molecules have also been implicated in SARS-CoV-2 infection, including the neuropilin-I receptor and CD147 (Zhang et al., 2020; Cantuti-Castelvetri et al., 2020; Wang et al., 2020). Additionally, the molecules CD209 and CD209L (DC-SIGN and L-SIGN, respectively) act as receptors for other human coronaviruses, such as NL-63 and SARS-CoV (Marzi et al., 2004). CD209L is expressed largely in type II alveolar cells and lung endothelial cells, much like ACE2, and shares almost 80% sequence homology with CD209. Therefore, the authors of a study from the Boston University Medical Center hypothesized that CD209L and CD209 may act as receptors for the novel coronavirus SARS-CoV-2.
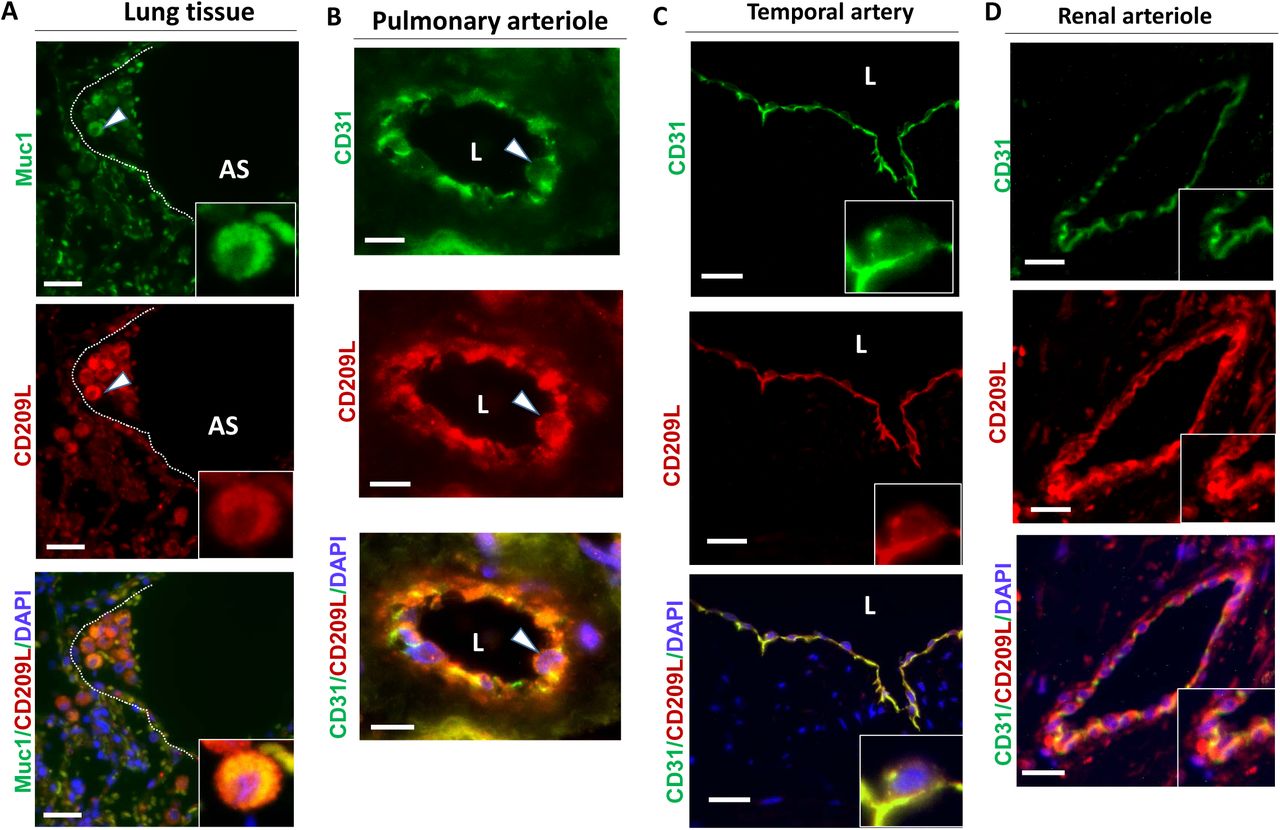
The authors first validated the expression profile of CD209 and CD209L in tissues known to be targeted by SARS-CoV-2: cells of the lung endothelium and epithelium, renal tubules and glomeruli, and the temporal artery in the central nervous system. When co-stained for CD209L and MUC1, a marker of type II alveolar cells, the lung tissue exhibited “prominent expression” of CD209L co-localized with MUC1. CD209L was similarly observed in pulmonary and renal arterioles, and in the endothelium of the temporal artery, where CD31 served as a marker of endothelial cells (Figure 1). CD209 was expressed only marginally in type II alveolar cells, and not at all in pulmonary or renal arterioles (data not shown). It was, however, expressed significantly in proximal tubular epithelial cells in the kidneys. Thus, the expression profiles of CD209 and CD209L overlap to a degree; but the expression of CD209L is more relevant to infection by SARS-CoV-2, given the similarity of its distribution to ACE2.
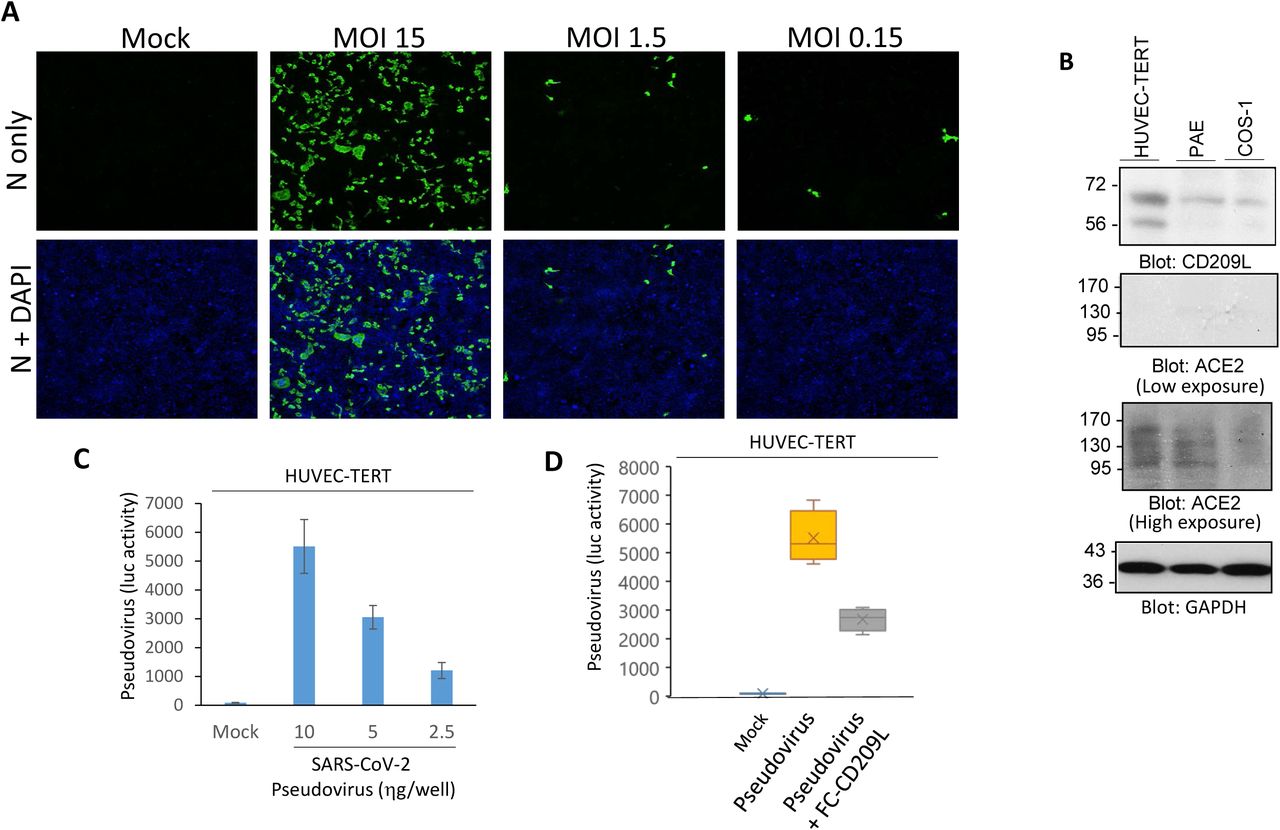
To determine the ability of SARS-CoV-2 to infect endothelial cells, HUVEC-TERT (human umbilical vein endothelial cells immortalized with TERT) were infected with SARS-CoV-2 at different multiplicities of infection (MOI). At all MOIs tested, viral particles were detected at 1 day post infection, which indicates the permissiveness of human endothelial cells to SARS-CoV-2 (Figure 2A). The authors next compared the expression of CD209L and ACE2 in several cell lines: HUVEC-TERT, PAE (porcine aortic endothelial cells), and COS-1 (non-endothelial transformed green monkey kidney cells). Of the three, HUVEC-TERT cells displayed the highest levels of CD209L, and COS-1 cells displayed the lowest (Figure 2B). Compared to the expression levels of CD209L, ACE2 expression was relatively low. Taken together, this may indicate CD209L as the primary route of infiltration of the vascular system in vivo.
Further, to assess the ability of CD209L to directly mediate SARS-CoV-2 entry in HUVEC-TERT cells, various concentrations of SARS-CoV-2 Spike protein-pseudotyped lentiviral particles were used to infect the endothelial cells. The magnitude of infection was determined by luciferase gene reporter assay in the cells. In a neutralization assay using the extracellular domain of CD209L fused to the Fc (constant) portion of an antibody, the soluble CD209L-Fc reduced viral entry by almost 50% (Figure 2C-D).
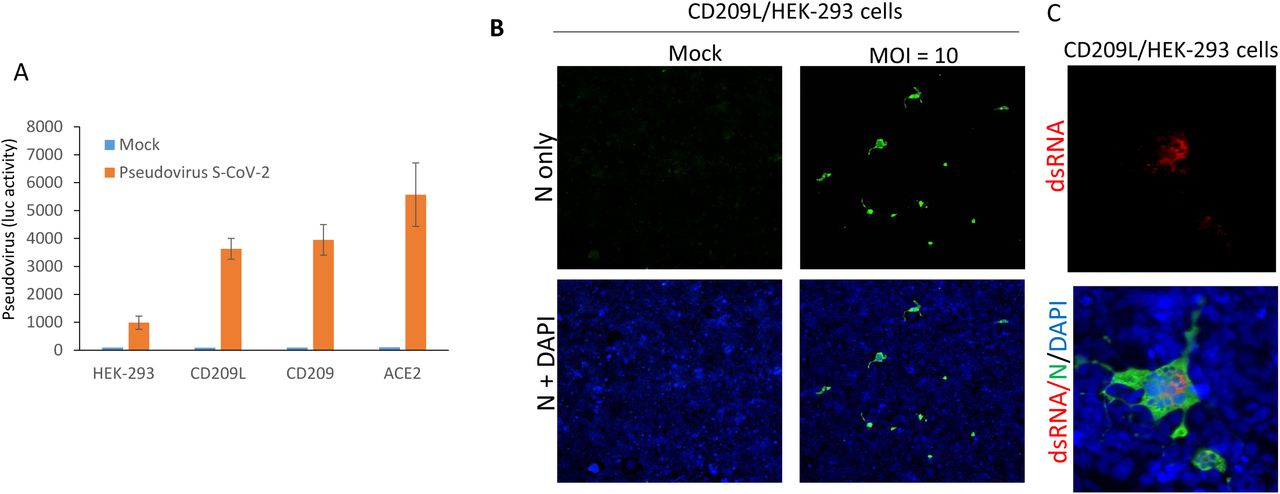
The authors next wanted to determine the specific roles of CD209 and CD209L in mediating SARS-CoV-2 entry into host cells. CD209, CD209L, and ACE2 were expressed in HEK-293 cells and infected with the SARS-CoV-2 pseudotyped lentiviral particles. They found that both CD209 and CD209L could facilitate pseudotyped viral entry, though both to a much lesser extent than could ACE2, as measured by luciferase reporter gene assay (Figure 3A). As in HUVEC-TERT endothelial cells, CD209L expression allowed SARS-CoV-2 entry into HEK-293 cells; active viral replication in these cells was indicated by the presence of double-stranded RNA molecules, as determined by immunofluorescence analysis (Figure 3B-C).
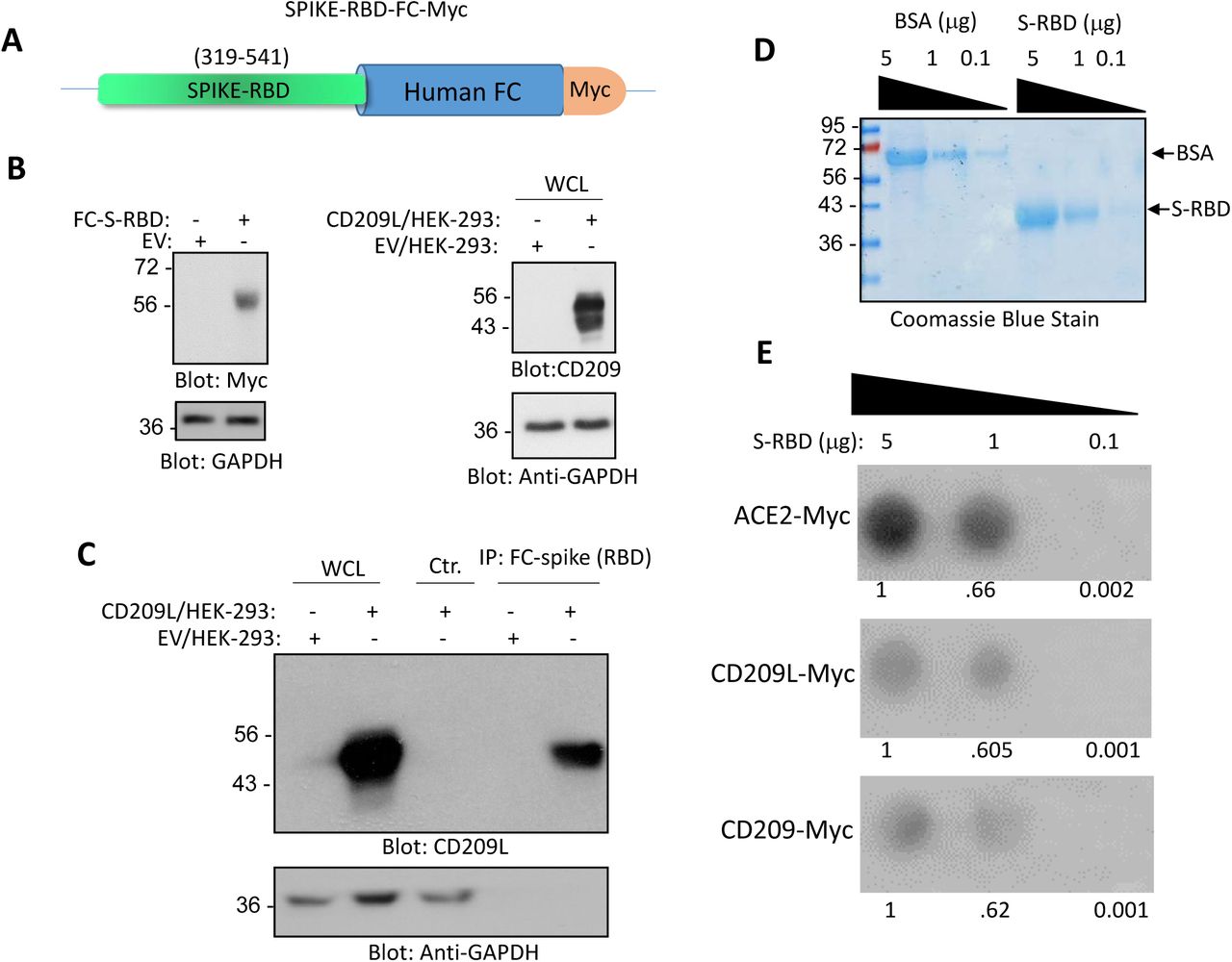
Finally, several immunoprecipitation assays were performed to investigate the specific mechanism by which CD209L mediates viral entry. In one assay, a chimeric soluble Fc-S-RBD-Myc protein co-precipitated with CD209L, indicating a specific interaction between the spike protein RBD (receptor binding domain) and CD209L (Figure 4A-B). As a follow-up, a dot blot assay was used to examine the binding of spike protein RBD with CD209, CD209L, and ACE2. As expected, the soluble SARS-CoV-2-S-RBD interacted with each of the molecules tested in a dose-dependent manner. ACE2 demonstrated the highest binding affinity to the RBD (Figure 4C-E).
In all, this work presents a novel route for SARS-CoV-2 infection and implicates the ability of the virus to infect systemically, not just via the lungs. The presence of CD209 and CD209L in the vascular endothelium and kidneys indicates the potential for varied sequelae that we have not yet encountered. This work also yields a new route for the development of vaccines against SARS-CoV-2. However, future work should focus on characterizing the relative involvement of each viral receptor across tissues, and how the use of one receptor over another by the virus results in differential outcomes in terms of the manifestation of COVID-19 and its severity.
References
Amraie, R., Napoleon, M. A., Yin, W., Berrigan, J., Suder, E., Zhao, G., Olejnik, J., Gummuluru, S., Muhlberger, E., Chitalia, V., & Rahimi, N. (2020). CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2 and are differentially expressed in lung and kidney epithelial and endothelial cells. BioRxiv, 2020.06.22.165803. https://doi.org/10.1101/2020.06.22.165803
Cantuti-Castelvetri, L., Ohja, R., Pedro, L., Djannatian, M., Franz, J., Kuivanen, S., Kallio, K., Kaya, T., Anastasina, M., Smura, T., Levanov, L., Szirovicza, L., Tobi, A., Kallio-Kokko, H., Osterlund, P., Joensuu, M., Meunier, F., Butcher, S., Winkler, M., … Simons, M. (2020). Neuropilin-1 facilitates SARS-CoV-2 cell entry and provides a possible pathway into the central nervous system. BioRxiv, 2020.06.07.137802. https://doi.org/10.1101/2020.06.07.137802
Marzi, A., Gramberg, T., Simmons, G., Möller, P., Rennekamp, A. J., Krumbiegel, M., Geier, M., Eisemann, J., Turza, N., Saunier, B., Steinkasserer, A., Becker, S., Bates, P., Hofmann, H., & Pöhlmann, S. (2004). DC-SIGN and DC-SIGNR Interact with the Glycoprotein of Marburg Virus and the S Protein of Severe Acute Respiratory Syndrome Coronavirus. Journal of Virology, 78(21), 12090–12095. https://doi.org/10.1128/jvi.78.21.12090-12095.2004
Wang, K., Chen, W., Zhou, Y.-S., Lian, J.-Q., Zhang, Z., Du, P., Gong, L., Zhang, Y., Cui, H.-Y., Geng, J.-J., Wang, B., Sun, X.-X., Wang, C.-F., Yang, X., Lin, P., Deng, Y.-Q., Wei, D., Yang, X.-M., Zhu, Y.-M., … Chen, Z.-N. (2020). SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. BioRxiv, 2020.03.14.988345. https://doi.org/10.1101/2020.03.14.988345
Zhang, H., Penninger, J. M., Li, Y., Zhong, N., & Slutsky, A. S. (2020). Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Medicine, 46(4), 586–590. https://doi.org/10.1007/s00134-020-05985-9
Leave a Reply