As some of the primary surveillance cells of the immune system, dendritic cells and macrophages are often the first immune cells to encounter pathogens in the body. Their role as antigen presenting cells means that they sit at a crucial hub in the initiation of the immune response; as a result, infecting agents have evolved mechanisms to subvert their action, and in some cases even infect these cells directly (Miller et al., 2008; Pollock et al., 1997; Bray, Geisbert, 2005; Wu, Kewal-Ramani, 2006). Coronaviruses are no exception: previous work has demonstrated the capacity that both SARS-CoV and MERS-CoV possess to infect and actively replicate inside macrophages and dendritic cells, in addition to triggering dysfunctional cytokine production (Law et al., 2005; Zhou et al., 2014). As in SARS, COVID-19 patients routinely present with heightened levels of proinflammatory cytokines – especially in severe cases – which may contribute to severity of the disease (Huang et al., 2020). This pathology of SARS-CoV-2 that mirrors that of SARS- and MERS-CoV has led researchers to hypothesize a potentially infectious interaction between the novel coronavirus and dendritic cells and macrophages. An interaction of this sort could be the source of any observed cytokine dysregulation. In a paper published earlier this summer in The Journal of Infectious Diseases, a Hong-Kong-based team investigated the viral and host cell responses to SARS-CoV-2 infection of monocyte-derived dendritic cells (moDCs) and monocyte-derived macrophages (MDMs).
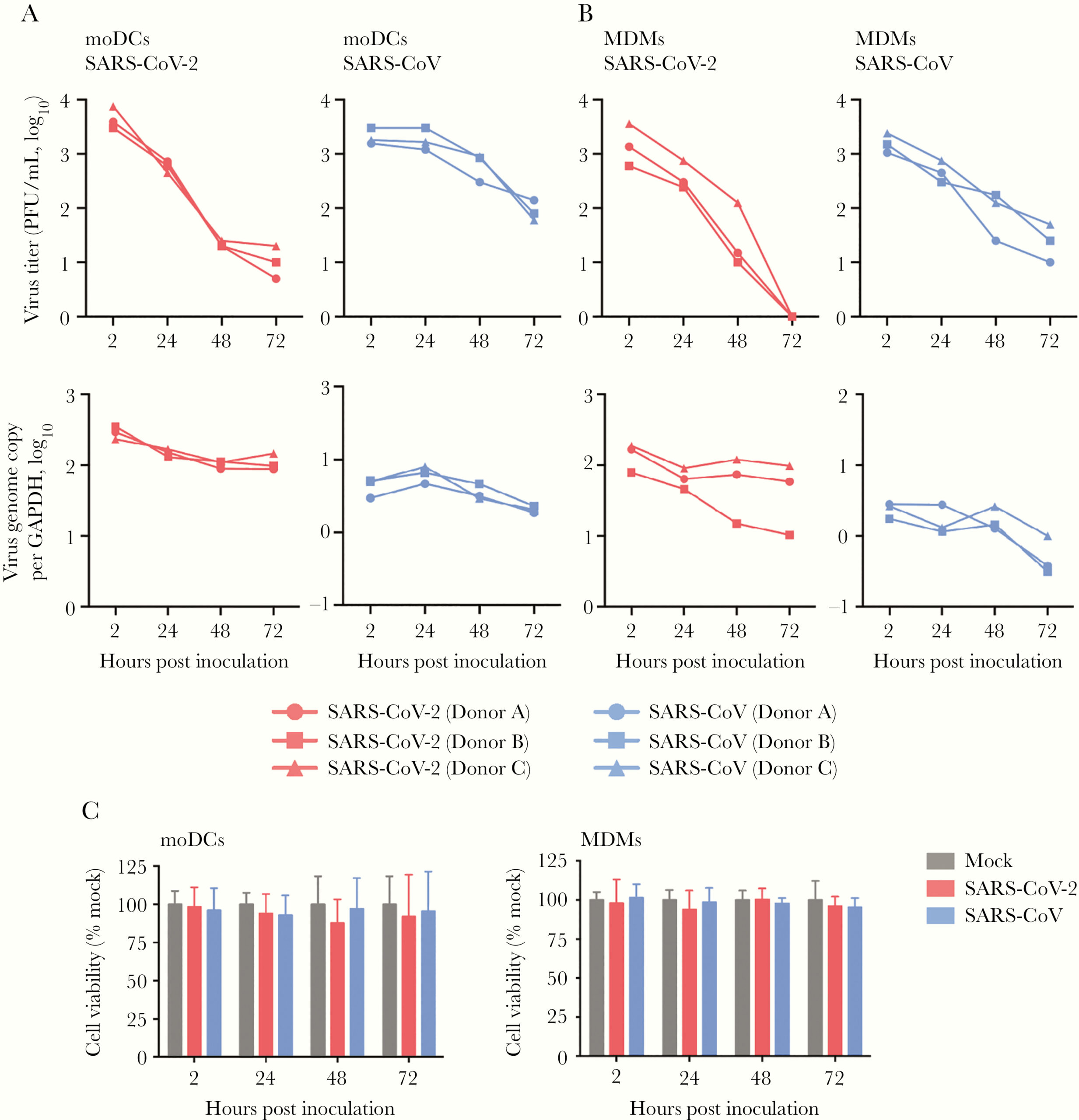
The first step was to determine the ability of SARS-CoV-2 to infect DCs and macrophages. To this end, the researchers collected peripheral blood mononuclear cells (PBMCs) from healthy donors, then separated and differentiated primary moDCs and MDMs from each sample. These cells were then infected with SARS-CoV-2 isolated from nasopharyngeal specimens of COVID-19 patients. In order to determine that infection had been successfully established in the human cells, immunofluorescence staining and confocal microscopy were used to evaluate the expression of the SARS-CoV-2 nucleocapsid (N) protein. They found that N protein was highly expressed in both moDCs and MDMs that were infected (Figure 1). The authors also noted significant cell-cell fusion in the infected moDCs (Figure 1). Together, this suggests efficient establishment of infection in human DCs and macrophages by SARS-CoV-2. Further investigation revealed that neither the viral titer nor viral genome copies from cell supernatants and lysates, respectively, increased over a period of 72 hours in either cell type (Figure 2A, 2B). The decrease in viral particulate inside the cells indicates that SARS-CoV-2 infection of these cells was abortive. Because previous work has demonstrated that MERS-CoV could induce apoptosis in T cells upon infection, the authors observed cell viability of moDCs and MDMs after infection with SARS-CoV-2 to determine whether the virus could induce similar effects (Chu et al., 2015). Using the CellTiterGlo assay, they noted a reduction of roughly 10% – 20% in moDCs at 72 hours post-infection, though this reduction was not statistically significant (Figure 2C). The viability of MDMs was essentially unaffected. In all, though moDCs and MDMs proved to be susceptible to infection, they do not efficiently support replication or the production of viral progeny.

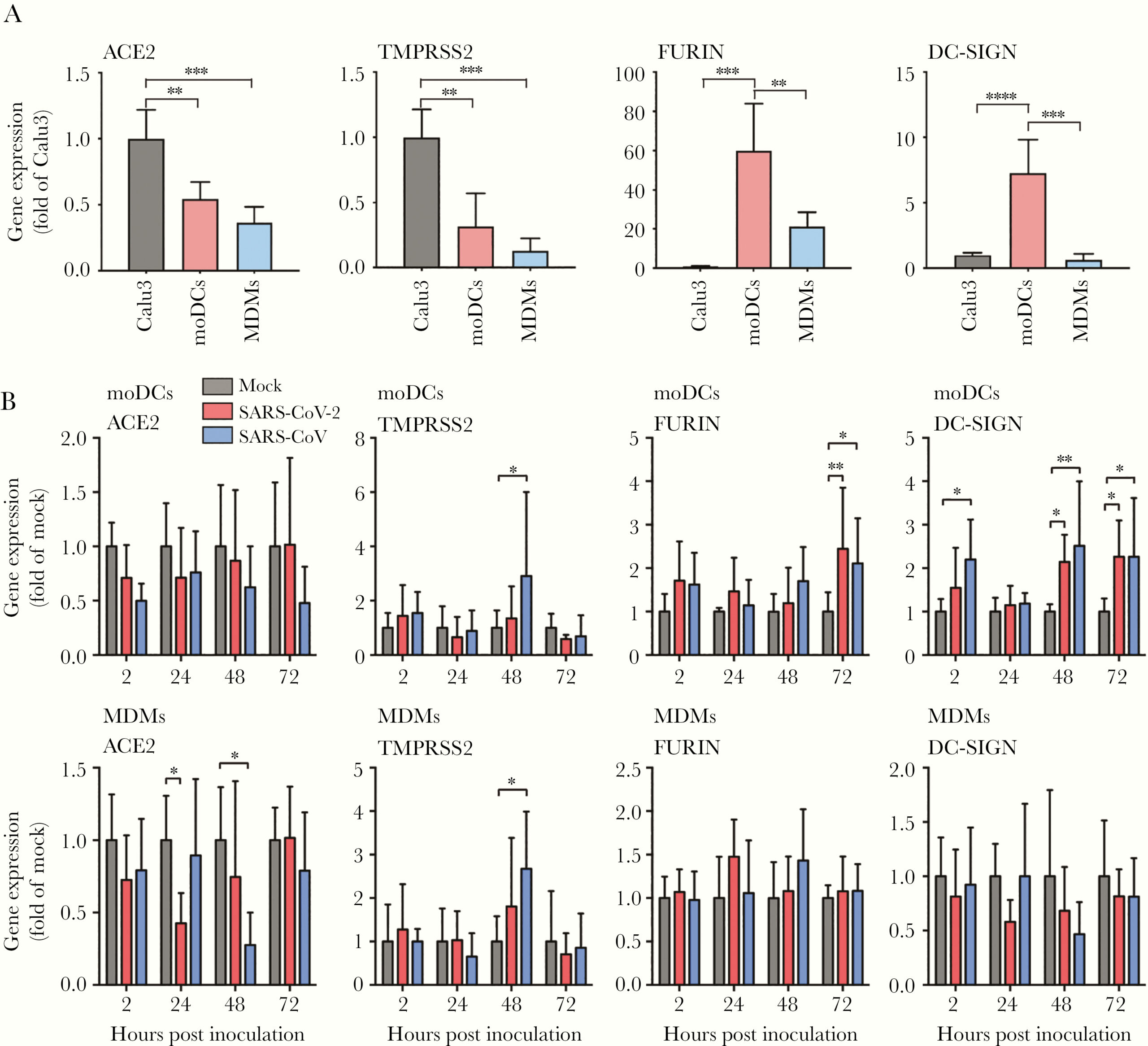
To replicate efficiently in host cells, viruses rely on interactions with various types of host proteins. In order to determine why SARS-CoV-2 was unable to replicate inside moDCs and MDMs, the authors investigated the expression of several host factors they believed may be involved in SARS-CoV-2 entry and replication: ACE2, TMPRSS2, FURIN, and DC-SIGN. Compared to the control cells – from the Calu3 cell line – uninfected moDCs and MDMs expressed less ACE2 and TMPRSS2, but significantly more FURIN; and moDCs expressed significantly more DC-SIGN than Calu3 cells (Figure 3A). When infected, the expression of ACE2 was significantly downregulated in MDMs, and the expression of both FURIN and DC-SIGN were significantly upregulated in moDCs (Figure 3B). Taken together, these data suggest that the inefficient replication of SARS-CoV-2 was not due to a lack of host factors in the DCs and macrophages.
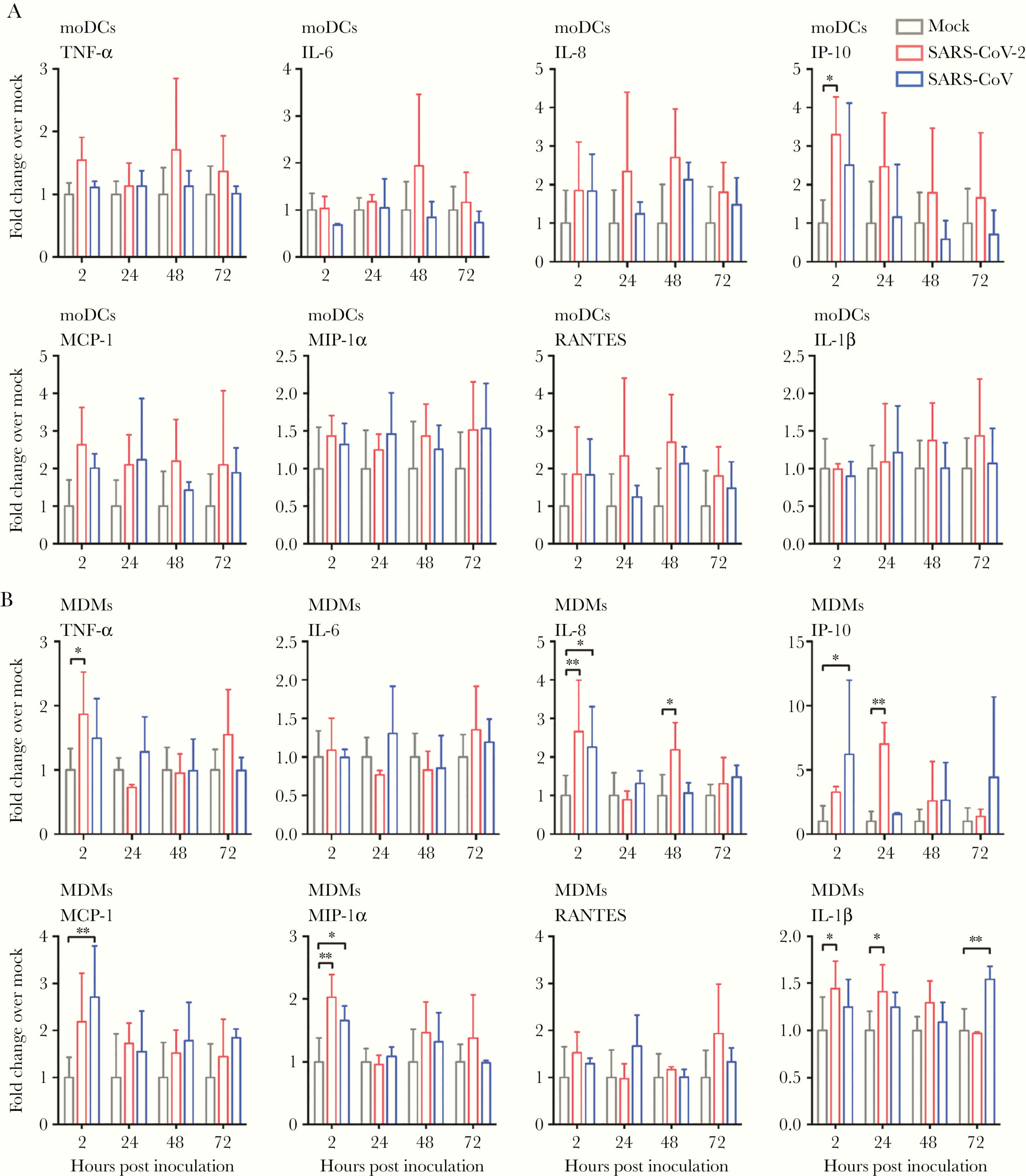
Next, the authors sought to characterize the production of interferon (IFN) and proinflammatory cytokines by moDCs and MDMs upon infection with SARS-CoV-2. They found that SARS-CoV-2 did not induce production of interferons of any type (I, II or III) from either cell type (Figure 4). In fact, the levels of IFNs type I and III were lower in SARS-CoV-2-infected MDMs than in mock-infected MDM controls. Additionally, infection did not significantly trigger expression of any proinflammatory cytokines or chemokines in moDCs, except for the molecule IP-10 (Figure 5A). In MDMs, however, SARS-CoV-2 infection significantly upregulated the expression of TNF-alpha, IL-8, IP-10, MIP-1-alpha, and IL-1-beta, representing five out of the eight cytokines/chemokines being tested. In these cells, IP-10 was the most upregulated of the five (Figure 5B). Cytokine storm is one of the major factors associated with disease severity in COVID-19. These data present a potential mechanism for the substantial cytokine release observed in even mild patients.

To the authors, the failure to activate IFN production indicated a dysregulation of the IFN signaling pathways induced by SARS-CoV-2. To investigate this, they measured the expression of the intracellular protein STAT1, a molecule involved in the activation of type I IFN, and the efficiency of STAT1 phosphorylation upon IFN-alpha treatment in the presence of absence of SARS-CoV-2 infection (phosphorylation of STAT1 activates the IFN signaling pathway). Upon infection, STAT1 expression was upregulated in moDCs, but not MDMs (Figure 6A). Additionally, the activation of STAT1 by IFN-alpha pretreatment was “significantly mitigated” by infection in moDCs (Figure 6B). SARS-CoV-2 did not appear to have any effect on STAT1 phosphorylation/activation in MDMs. Altogether, these data suggest that SARS-CoV-2 infection causes dysregulation of IFN production via inhibition of STAT1 phosphorylation in moDCs. The data presented in this paper provide insight into the interaction between SARS-CoV-2 and dendritic cells and macrophages, information that may help guide treatment or the development of therapies and vaccines down the line.

References
Bray, M., & Geisbert, T. W. (2005). Ebola virus: The role of macrophages and dendritic cells in the pathogenesis of Ebola hemorrhagic fever. In International Journal of Biochemistry and Cell Biology (Vol. 37, Issue 8, pp. 1560–1566). Elsevier Ltd. https://doi.org/10.1016/j.biocel.2005.02.018
Chu, H., Zhou, J., Wong, B. H. Y., Li, C., Chan, J. F. W., Cheng, Z. S., Yang, D., Wang, D., Lee, A. C. Y., Li, C., Yeung, M. L., Cai, J. P., Chan, I. H. Y., Ho, W. K., To, K. K. W., Zheng, B. J., Yao, Y., Qin, C., & Yuen, K. Y. (2016). Middle East Respiratory Syndrome Coronavirus Efficiently Infects Human Primary T Lymphocytes and Activates the Extrinsic and Intrinsic Apoptosis Pathways. Journal of Infectious Diseases, 213(6), 904–914. https://doi.org/10.1093/infdis/jiv380
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., Zhang, L., Fan, G., Xu, J., Gu, X., Cheng, Z., Yu, T., Xia, J., Wei, Y., Wu, W., Xie, X., Yin, W., Li, H., Liu, M., … Cao, B. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet, 395(10223), 497–506. https://doi.org/10.1016/S0140-6736(20)30183-5
Kwaik, Y. A., Eisenstein, B. I., & Engleberg, N. C. (1993). Phenotypic modulation by Legionella pneumophila upon infection of macrophages. Infection and Immunity, 61(4), 1320–1329. https://doi.org/10.1128/iai.61.4.1320-1329.1993
Law, H. K. W., Chung, Y. C., Hoi, Y. N., Sin, F. S., Yuk, O. C., Luk, W., Nicholls, J. M., Peiris, J. S. M., & Lau, Y. L. (2005). Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood, 106(7), 2366–2374. https://doi.org/10.1182/blood-2004-10-4166
Miller, J. L., DeWet, B. J. M., Martinez-Pomares, L., Radcliffe, C. M., Dwek, R. A., Rudd, P. M., & Gordon, S. (2008). The mannose receptor mediates dengue virus infection of macrophages. PLoS Pathogens, 4(2), e17. https://doi.org/10.1371/journal.ppat.0040017
Pollock, J. L., Presti, R. M., Paetzold, S., & Virgin IVth, H. W. (1997). Latent murine cytomegalovirus infection in macrophages. Virology, 227(1), 168–179. https://doi.org/10.1006/viro.1996.8303
Wu, L., & Kewal-Ramani, V. N. (2006). Dendritic-cell interactions with HIV: Infection and viral dissemination. In Nature Reviews Immunology (Vol. 6, Issue 11, pp. 859–868). Nature Publishing Group. https://doi.org/10.1038/nri1960
Yang, D., Chu, H., Hou, Y., Chai, Y., Shuai, H., Lee, A. C. Y., Zhang, X., Wang, Y., Hu, B., Huang, X., Yuen, T. T. T., Cai, J. P., Zhou, J., Yuan, S., Zhang, A. J., Chan, J. F. W., & Yuen, K. Y. (2020). Attenuated Interferon and Proinflammatory Response in SARS-CoV-2-Infected Human Dendritic Cells Is Associated With Viral Antagonism of STAT1 Phosphorylation. The Journal of Infectious Diseases, 222(5), 734–745. https://doi.org/10.1093/infdis/jiaa356
Zhou, J., Chu, H., Li, C., Wong, B. H. Y., Cheng, Z. S., Poon, V. K. M., Sun, T., Lau, C. C. Y., Wong, K. K. Y., Chan, J. Y. W., Chan, J. F. W., To, K. K. W., Chan, K. H., Zheng, B. J., & Yuen, K. Y. (2014). Active replication of middle east respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: Implications for pathogenesis. Journal of Infectious Diseases, 209(9), 1331–1342. https://doi.org/10.1093/infdis/jit504
Leave a Reply