Recently, findings of the phase I part of a phase I–II trial of Novavax’s vaccine candidate, which began in May 2020, were released in the New England Journal of Medicine. The vaccine candidate, called NVX-CoV2373, is a recombinant SARS-CoV-2 nanoparticle vaccine created from the full-length, wild-type, virus spike (S) glycoprotein which mediates viral entry in the host cell. The first of its type to undergo human trials for SARS-CoV-2.
The primary aim of this randomized, placebo-controlled, trial (which enrolled healthy adult volunteers under the age of 60 from two locations in Australia) was to evaluate the safety and immunogenicity of the vaccine at two doses, 5-μg and 25-μg, with or without Matrix-M1 adjuvant (50-μg dose). Investigators reported high immune responses with relatively mild side effects in participants experiencing adverse events. Furthermore, after the second vaccination of patients that received both the vaccine and adjuvant, serum antibody levels observed were several times greater than levels from samples of hospitalized patients with Covid-19.
Study Background and Results
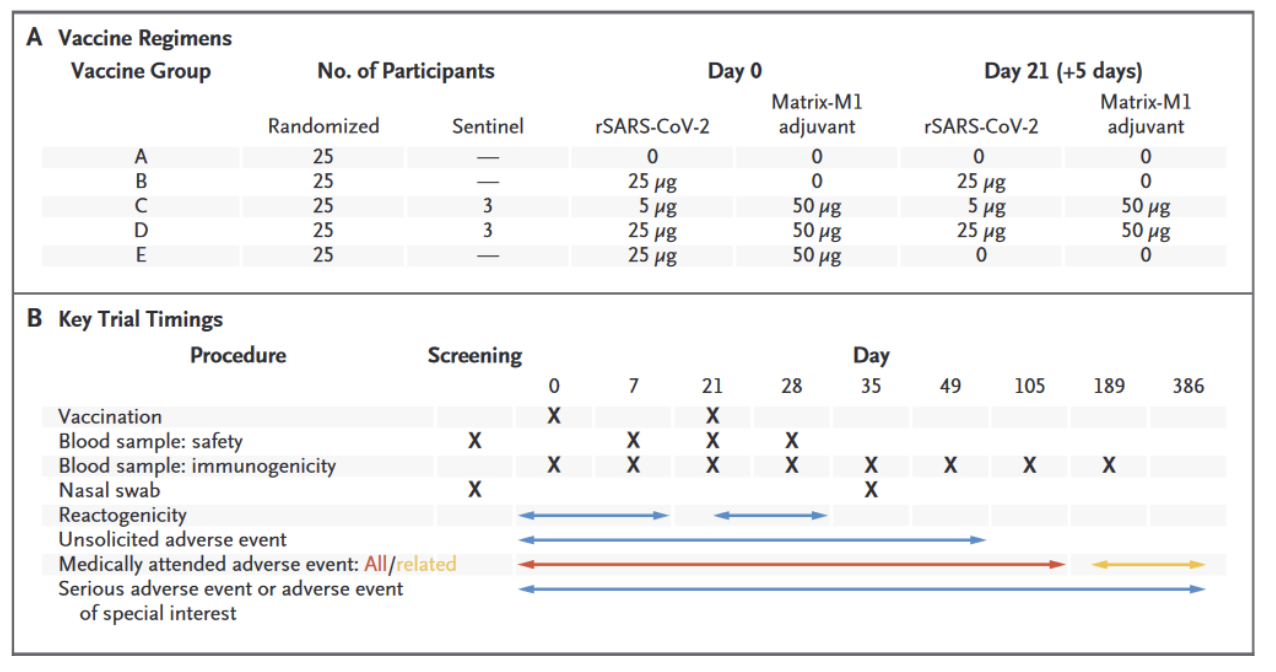
This randomized, placebo-controlled, trial consisted of five experimental arms that measured the response of the vaccine at two different doses: 5 μg, 20 μg. NVX-CoV2373 was administered as two 0.6-ml intramuscular (IM) injections in the deltoid muscle 21 days apart. Furthermore, as a safety measure 6 participants were initially randomly assigned to groups C and D (5-μg and 25-μg dose groups plus Matrix-M) and observed for reactogenicity for 48 hours. Subsequently, the remaining participants were randomly assigned, in a blinded manner, to one of five vaccine groups without stratification. Breakdown of the experimental set up of the study are shown in Figure 1.
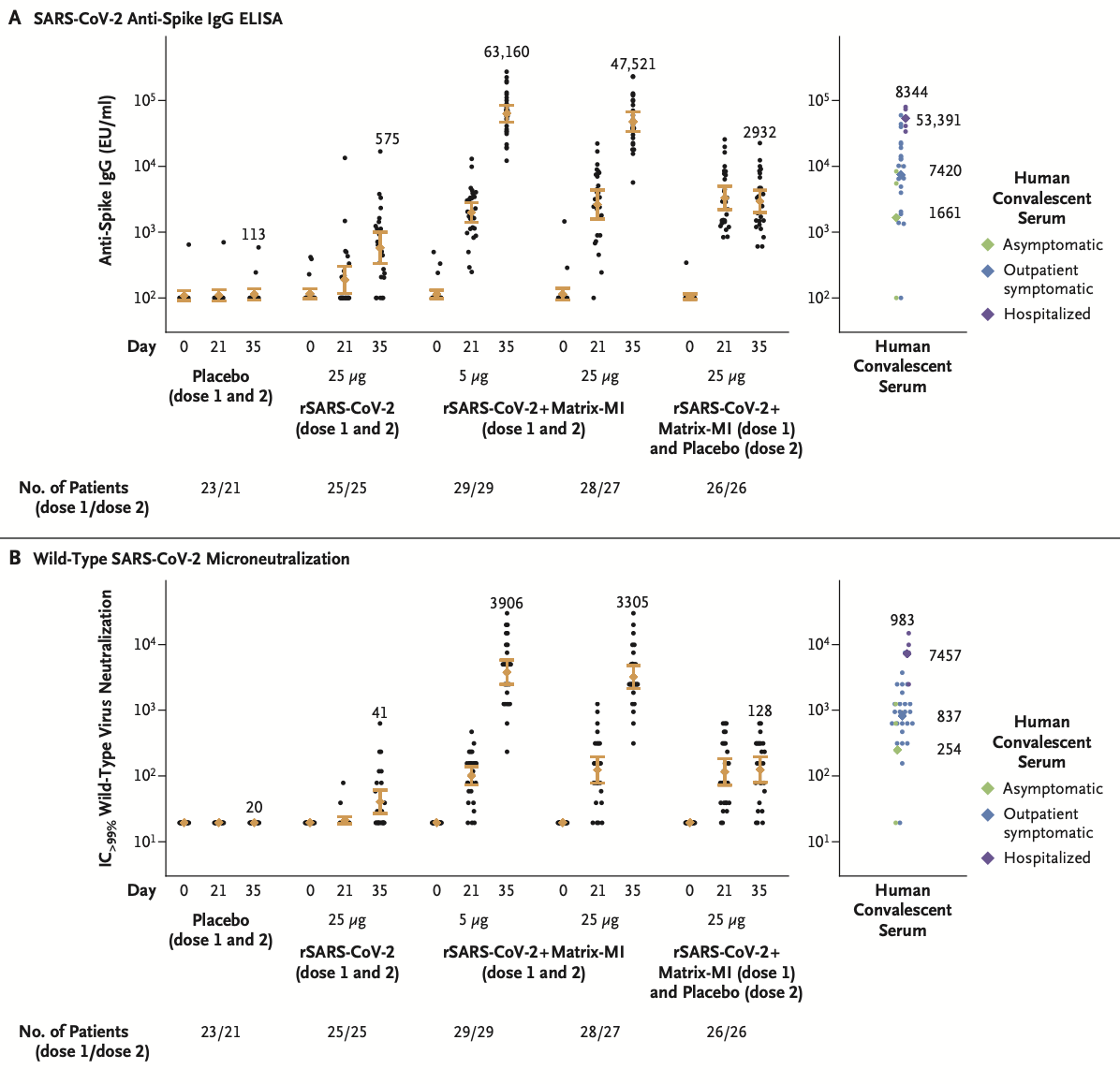
Assessment of SARS-CoV-2 neutralizing antibodies was carried out by enzyme-linked immunosorbent assay (ELISA) and microneutralization assay at an inhibitory concentration greater than 99% (Figure 2). In addition, the investigators used a panel consisting of 32 (IgG) and 29 (MN) convalescent serum specimens collected from patients with PCR-confirmed Covid-19, and classified by severity of symptoms, as controls. The results showed a robust immune response in all participants, especially those patients receiving both the vaccine and adjuvant regimen. Moreover, a second vaccination with adjuvant correlated with titer levels exceeding those in convalescent serum from symptomatic outpatients with Covid-19 by a factor of at least 6 (rising to levels comparable with convalescent serum samples from patients hospitalized with Covid-19). Interestingly, responses in both dose regimens with adjuvant were similar, which points to the necessity of adjuvant use in the formulation of NVX-CoV2373.
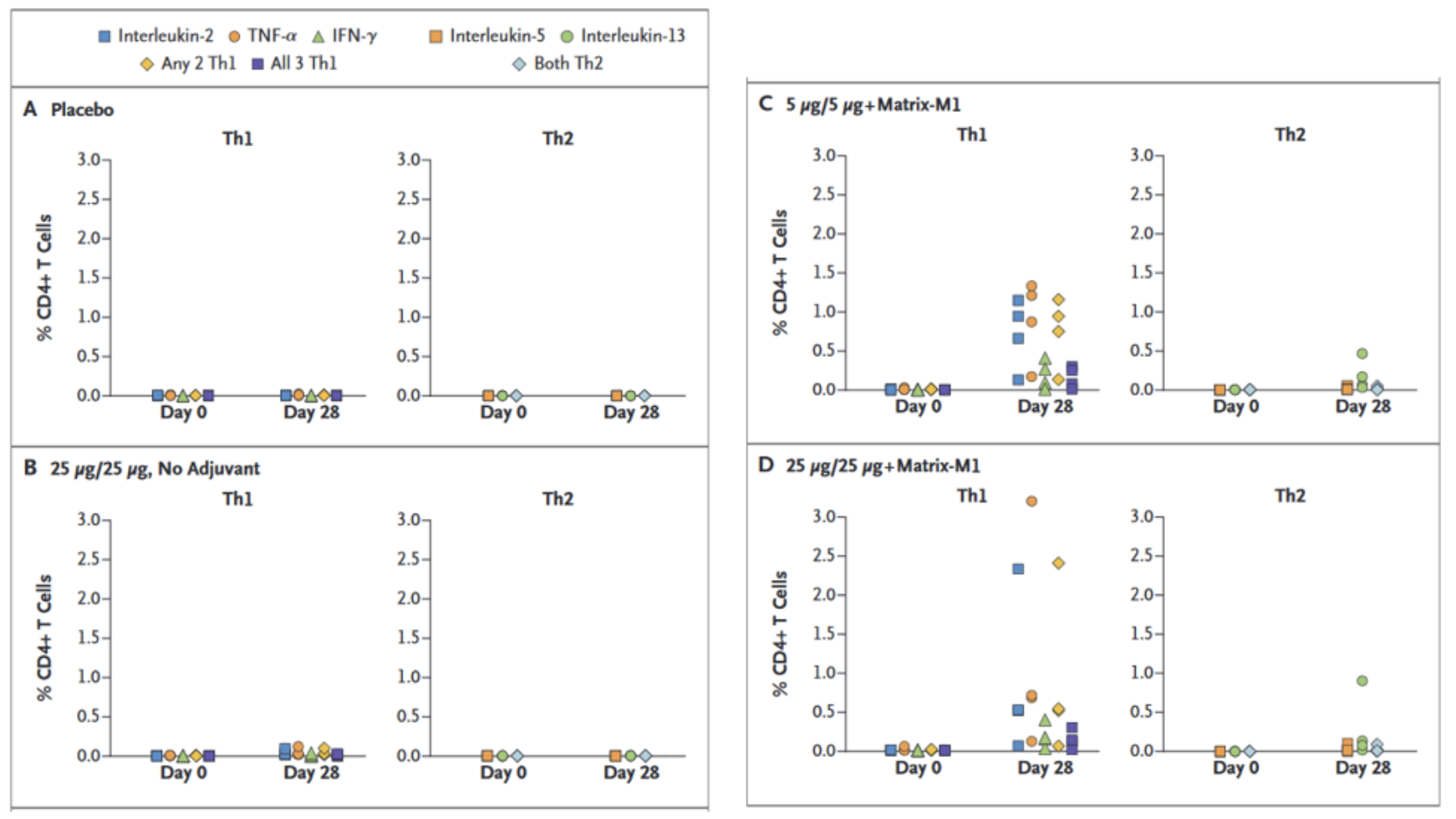
T-Cell response assessment against SARS-CoV-2 spike protein was carried out by intracellular cytokine staining assay. The assay results, Figure 3, give an insight into the immune response generated by vaccine administration. Interestingly, the CD4 T-Cell assay indicates a stronger expression of Th1 phenotype by the markedly higher expression of Th1 cytokine profile. Th2 responses (as measured by IL-5 and IL-13 cytokines) were minimal.
Lastly, there were no reports of serious adverse events with the administration of NVX-CoV2373 (however there were several participants that severe systemic events consisted mainly of joint pain and fatigue). The more common adverse events reported were fatigue, chills, headache, myalgia, and pain at the injection site. However, the majority of these instances were mild-moderate and most subsided after the second dose administration.
Last Remarks
The positive results of this trial add on to the emerging data of probable candidates to fight the COVID-19 pandemic. In particular, positive results from this type of vaccination might prove advantageous, both in terms of approval and further investigation, due to similar products having already been approved to treat infectious diseases such as shingles. Further data from the phase II part of the trial, which will comprise a larger sample of participants, will help clarify the efficacy of NVX-CoV2373 against SARS-CoV-2.
References
- Keech C, Albert G, et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N Engl J Med. Published online on September 2, 2020. doi: 10.1056/NEJMoa2026920
Leave a Reply