Last week, the Trump administration released guidelines regarding the distribution strategy for a COVID-19 vaccine through the U.S. Department of Health and Human Services (HHS) and the Department of Defense (DoD). These guidelines, found in this link, detail the American government’s plan to safely and effectively distribute a vaccine ahead of time as many of the developers in the market are still working to get their candidate approved (with the first yet to be approved in the U.S. as the time of this report). In addition, the guidelines assure that the administration aims to provide the vaccine with, “no upfront costs to providers and no out-of-pocket cost to the vaccine recipient.”
The document details the following four key tasks to achieve complete access to the American public as part of Operation Warp Speed:
- “Continue engaging with state, tribal, territorial, and local partners, other stakeholders, and the public to communicate public health information, before and after distribution begins, around the vaccine and promote vaccine confidence and uptake.
- Distribute vaccines immediately upon granting of Emergency Use Authorization/ Biologics License Application, using a transparently developed, phased allocation methodology.
- Ensure safe administration of the vaccine and availability of administration supplies.
- Monitor necessary data from the vaccination program through an information technology (IT) system capable of supporting and tracking distribution, administration, and other necessary data.”
Notably, the strategy to deliver the vaccine to administration sites consists of two phases. Phase I will be restricted to sites that are equipped to meet the early requirements for storage and handling of vaccine products, possibly due to constrictive stability requirements of some of the current vaccine candidates such as Moderna’s mRNA vaccine which is one of the top contenders at this moment. During Phase II, the guidelines allude to the eventual expansion of distribution to healthcare facilities such as adult and pediatric healthcare sites and pharmacies. The guidelines assure that, “the program will make maximum use of all healthcare professionals licensed to administer vaccines, including allied health professionals such as pharmacists.”
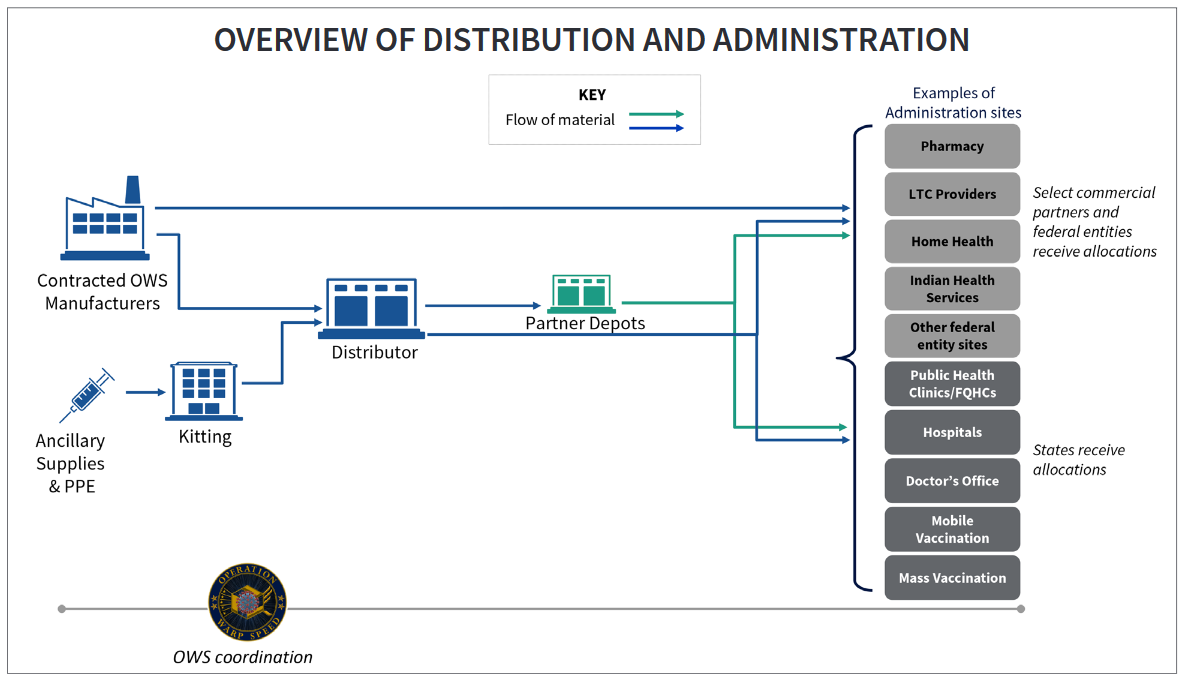
In addition, earlier this month the National Academies of Sciences Engineering and Medicine (NASEM), commissioned by the Centers for Disease Control and the National Institutes of Health, published a draft framework to discuss the equitable allocation of the COVID-19 vaccine as it becomes available (link here). The first phases of the plan allocates the earliest vaccinations to healthcare workers, as well as medically vulnerable groups, with the remaining phases allocating the vaccines to workers in essential industries.

As the pandemic continues, it is imperative that governments around the world strategically plan to deliver the first round of vaccines as they become available in the coming months or early next year. The distribution networks need to be staged in order for the vaccine product to be safe to use, as well as made available to vulnerable populations first. Unprecedented times call for unprecedented action!
References
- U.S. Department of Health and Human Services. (2020, September 16). Trump Administration Releases COVID-19 Vaccine Distribution Strategy. Retrieved September 23, 2020, from https://www.hhs.gov/about/news/2020/09/16/trump-administration-releases-covid-19-vaccine-distribution-strategy.html
- Subbaraman, N. (2020, September 17). Who gets a COVID vaccine first? Access plans are taking shape. Retrieved September 23, 2020, from https://www.nature.com/articles/d41586-020-02684-9
Leave a Reply