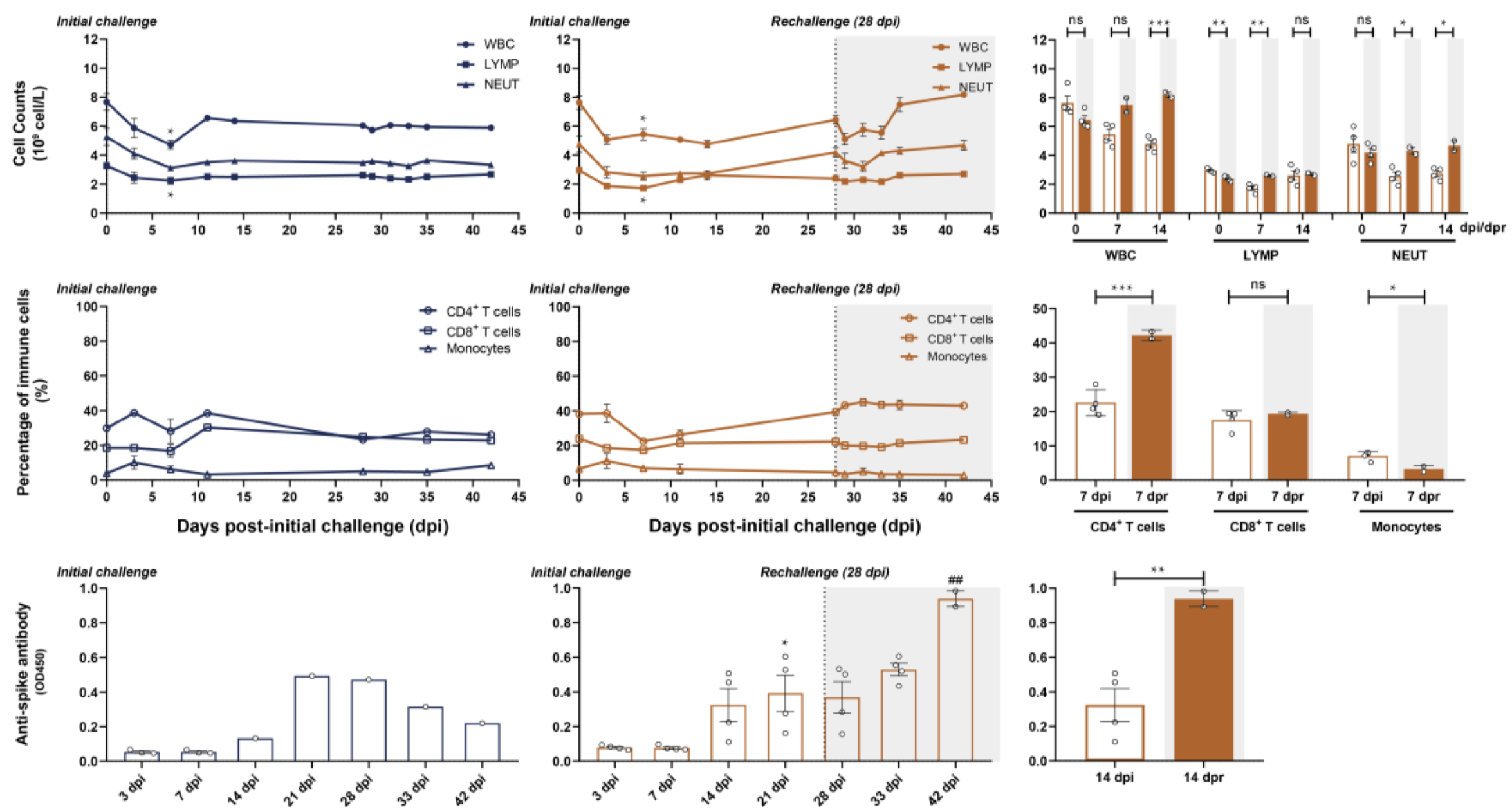
In an effort to determine the SARS-CoV-2-specific antibody response longitudinally, a team of researchers infected rhesus macaques with the virus and measured the viral load and antibody titer over the course of several weeks. Initially, six macaques were administered intratracheal injections of SARS-CoV-2 viral particles. At 28 days post infection (dpi, equal to 0 days post re-challenge [dpr]), four of the six were re-challenged intratracheally with the same viral dose; various cell and antibody counts were carried out to 45 dpi (17 dpr, Figure 1). In the primary challenge phase, peak viral load was detected by nasal and pharyngeal swabs at 3 dpi, and subsequently declined to undetectable levels at 14 dpi. Of the two macaques that were not given a secondary viral challenge, SARS-specific antibodies peaked at 21 dpi at a level that was significantly higher than the titer at 3 dpi. White blood cell (WBC), total lymphocyte, and neutrophil counts were elevated in the secondary challenged monkeys compared to the primary challenged monkeys; this difference reached significance by 14 dpi. Additionally, CD4+ T cells were significantly elevated in secondary challenged monkeys at 7 dpr compared to primary challenged monkeys at 7 dpi (Bao et al., 2020). Overall, these data demonstrate a potential build-up of specific neutralizing antibodies and immune cell response within a few weeks of viral infection that may serve ultimately to prevent reinfection by the same pathogen. There are several limitations to note: first, the antibody titers measured were not assessed for neutralizing capacity, so the anti-spike protein antibody titers may not necessarily accurately represent an infection-inhibiting immune response. Second, because neutrophils and monocytes are not antigen-specific, their presence is not necessarily indicative of a SARS-CoV-2-specific immune response. Similarly, enhanced antibody production may simply be the product of a cumulative immune response, rather than a signature of re-infection. To determine the precise mechanism, it will be useful in future studies to expand the course of the study, such that re-infection occurs far enough post-primary infection that the secondary immune response can be qualitatively and quantitatively differentiated from the initial response.
The hormones epinephrine (adrenaline), norepinephrine (noradrenaline), and dopamine comprise a class of molecules known as catecholamines. The catecholamines occupy various niches in the central and peripheral nervous systems where they act to modify behavior and as signaling molecules between neurons (Axelrod, Weinshilboum, 1972; Lotharius, Brundin, 2002; Hirasawa et al., 1993). Cytokine release syndrome (CRS) is a condition characterized by the hypersecretion of pro-inflammatory cytokines in response to antigenic stimulation and is often accompanied by a surge in catecholamines, some of which have been shown to be secreted directly by immune cells (Flierl et al., 2007). These catecholamines act in a feed-forward loop to augment the production of pro-inflammatory cytokines, which occurs primarily via the α1-type adrenergic receptor (α1-AR).
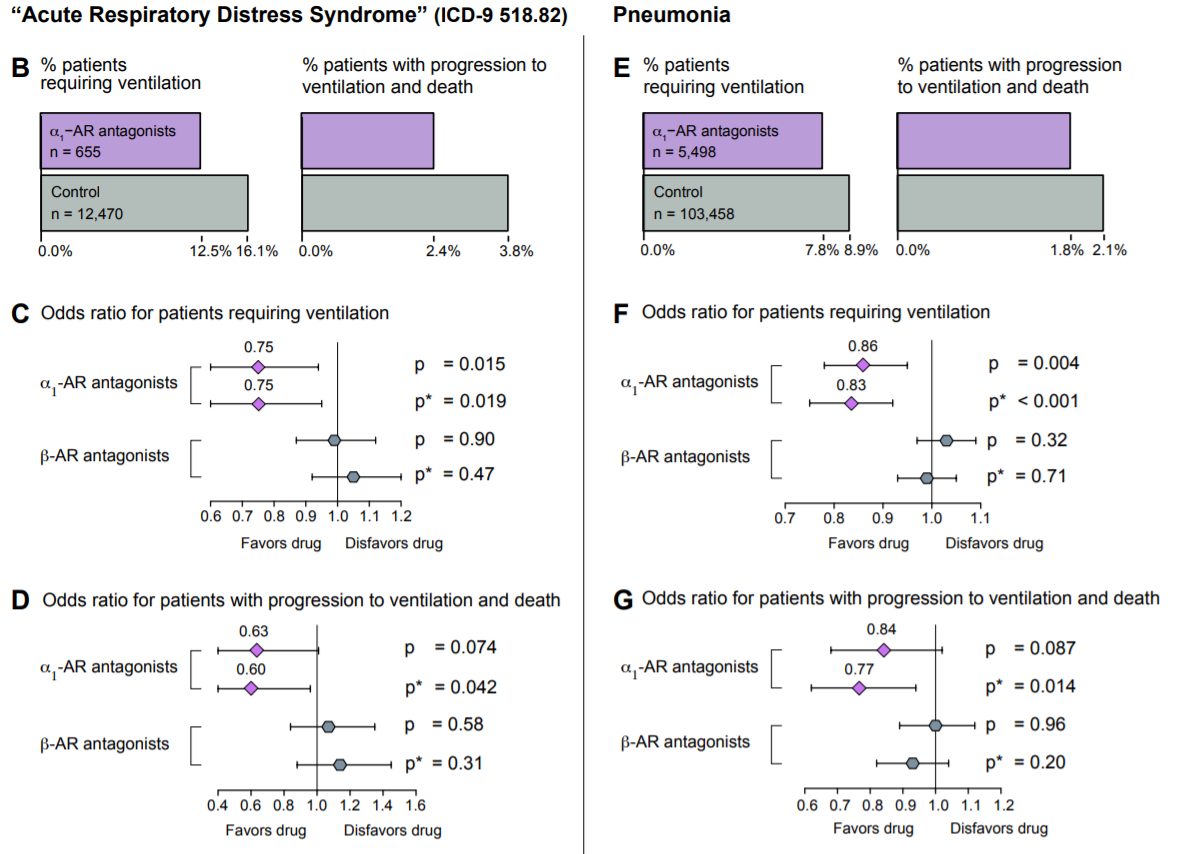
In a paper published earlier this month, Konig et al. analyzed the efficacy of α1-AR antagonists as protective agents in acute respiratory distress syndrome (ARDS) and pneumonia, two conditions that are almost ubiquitously observed in COVID-19. In this retrospective study, 122,081 patients from the MarketScan Research Database were split into an ARDS cohort and a pneumonia cohort, and were screened for α1-AR antagonist use and analyzed for one of two outcomes: progression to ventilation, or further progression to death while ventilated. Due to the retrospective nature of the study, α1-AR antagonist use was defined as “patients having filled [an] α1-AR antagonist prescription…in the year preceding [diagnosis of ARDS or pneumonia] for more than an aggregate of 180 days” (these patients would have been prescribed an antagonist for the treatment of various other chronic conditions that are not related to pneumonia or ARDS, conditions such as benign prostatic hyperplasia or hypertension). Of the 13,125 patients in the ARDS cohort, α1-AR antagonist users had a roughly 22% lower incidence of invasive mechanical ventilation compared to non-users and a roughly 36% lower incidence of further progression to death in the hospital while ventilated. In contrast, prior use of β-AR antagonists was not correlated with either outcome. Similarly, of the 108,956 patients in the pneumonia cohort, α1-AR antagonist users exhibited a roughly 13% lower incidence of invasive mechanical ventilation compared to non-users, as well as an approximately 16% lower incidence of further progression to death in the hospital on ventilation. Again, prior use of β-AR antagonists was not correlated with either outcome (Konig et al., 2020). It is important to note that though this study did not examine COVID-19 patients, it has vast implications for the use of catecholamine-interfering treatments in COVID-19, and suggests a possible path for the amelioration of the hyperinflammatory symptoms observed in these patients.
Written by: Parker Davis
Edited by: Jina Zhou and Esther Melamed
5/25/2020
References
Axelrod, J., & Weinshilboum, R. (1972). Catecholamines. In New England Journal of Medicine (Vol. 287, Issue 5, pp. 237–242). Massachusetts Medical Society. https://doi.org/10.1056/NEJM197208032870508
Bao, L., Deng, W., Gao, H., Xiao, C., Liu, J., Xue, J., Lv, Q., Liu, J., Yu, P., Xu, Y., Qi, F., Qu, Y., Li, F., Xiang, Z., Yu, H., Gong, S., Liu, M., Wang, G., Wang, S., … Qin, C. (2020). Reinfection could not occur in SARS-CoV-2 infected rhesus macaques. BioRxiv, 2020.03.13.990226. https://doi.org/10.1101/2020.03.13.990226
Flierl, M. A., Rittirsch, D., Nadeau, B. A., Chen, A. J., Sarma, J. V., Zetoune, F. S., McGuire, S. R., List, R. P., Day, D. E., Hoesel, L. M., Gao, H., Van Rooijen, N., Huber-Lang, M. S., Neubig, R. R., & Ward, P. A. (2007). Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature, 449(7163), 721–725. https://doi.org/10.1038/nature06185
Hírasawa, A., Horie, K., Tanaka, T., Takagaki, K., Murai, M., Yano, J., & Tsujimoto, G. (1993). Cloning, Functional Expression and Tissue Distribution of Human cDNA for the α1C-Adrenergic Receptor. Biochemical and Biophysical Research Communications, 195(2), 902–909. https://doi.org/10.1006/bbrc.1993.2130
Konig, M. F., Powell, M., Staedtke, V., Bai, R.-Y., Thomas, D. L., Fischer, N., Huq, S., Khalafallah, A. M., Koenecke, A., Papadopoulos, N., Kinzler, K. W., Vogelstein, B., Vogelstein, J. T., Athey, S., Zhou, S., & Bettegowda, C. (2020). Targeting the catecholamine-cytokine axis to prevent SARS-CoV-2 cytokine storm syndrome. MedRxiv, 2, 2020.04.02.20051565. https://doi.org/10.1101/2020.04.02.20051565
Lotharius, J., & Brundin, P. (2002). Pathogenesis of parkinson’s disease: Dopamine, vesicles and α-synuclein. Nature Reviews Neuroscience, 3(12), 932–942. https://doi.org/10.1038/nrn983
Leave a Reply