This week, there has been news for both remdesivir and chloroquine/ hydroxychloroquine. Following the announcement of preliminary results from the Gilead Science trials at the University of Chicago, there has been thought to be a promising potential in remdesivir with the trial now rapidly expanding.
There has also been further speculation on preemptively released results from a recent Chinese clinical trial, although the study was underpowered compared to other trials. In addition, this study’s design allowed for late enrollment of patients up to 12 days after symptom onset, at which point the viral load may already be peaking and antivirals such as remdesivir likely would not work at their full potential.
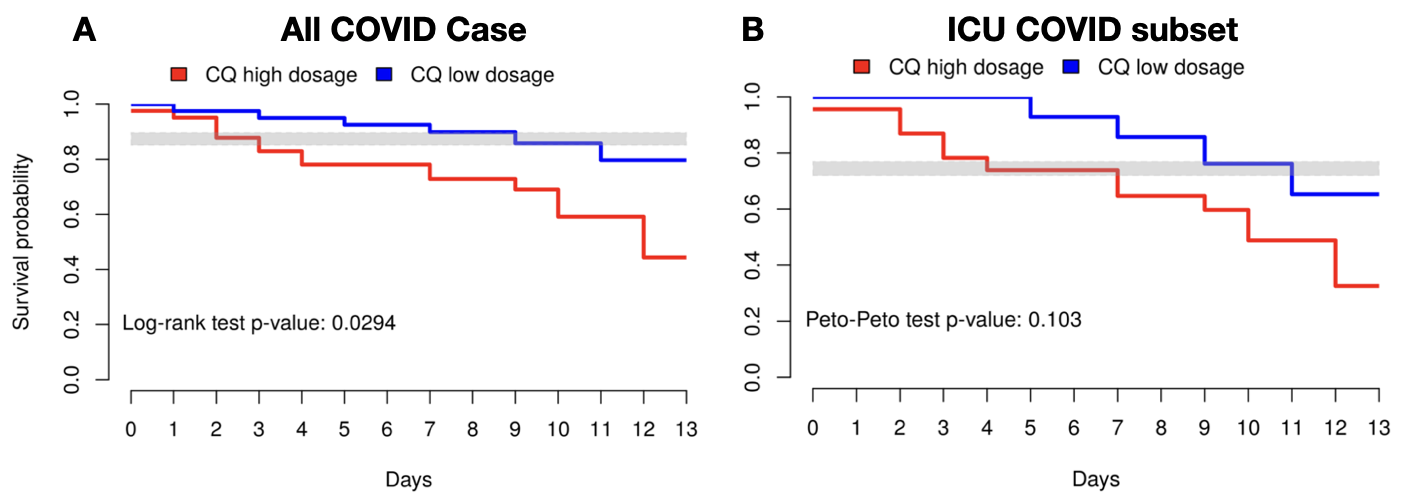
One of the first papers outlining patient health outcomes from chloroquine was released with a previously under discussed topic of dosage (Borba et al.). This study used both a high and low dose of chloroquine, which showed no clinical health outcome differences for low-dose chloroquine versus standard care and worse outcomes for high-dose chloroquine therapies (Figure 1A, Borba et al.). This trend also extended to the subset of patients who had critical cases and placement in the ICU (Figure 1B, Borba et al.). As a result, this trial had to end prematurely due to the detriment of the high dosage.
References
Borba MGS, Val FFA, Sampaio VS, et al. Effect of High vs Low Doses of Chloroquine Diphosphate as Adjunctive Therapy for Patients Hospitalized With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection: A Randomized Clinical Trial. JAMA Netw Open. 2020;3(4.23):e208857. doi:10.1001/jamanetworkopen.2020.8857
Leave a Reply