Summary: Results of a phase 1 trial have revealed that the Covid-19 vaccine developed by the US-based biotechnology company Moderna elicited a strong immune response in older adults with no serious adverse effects.
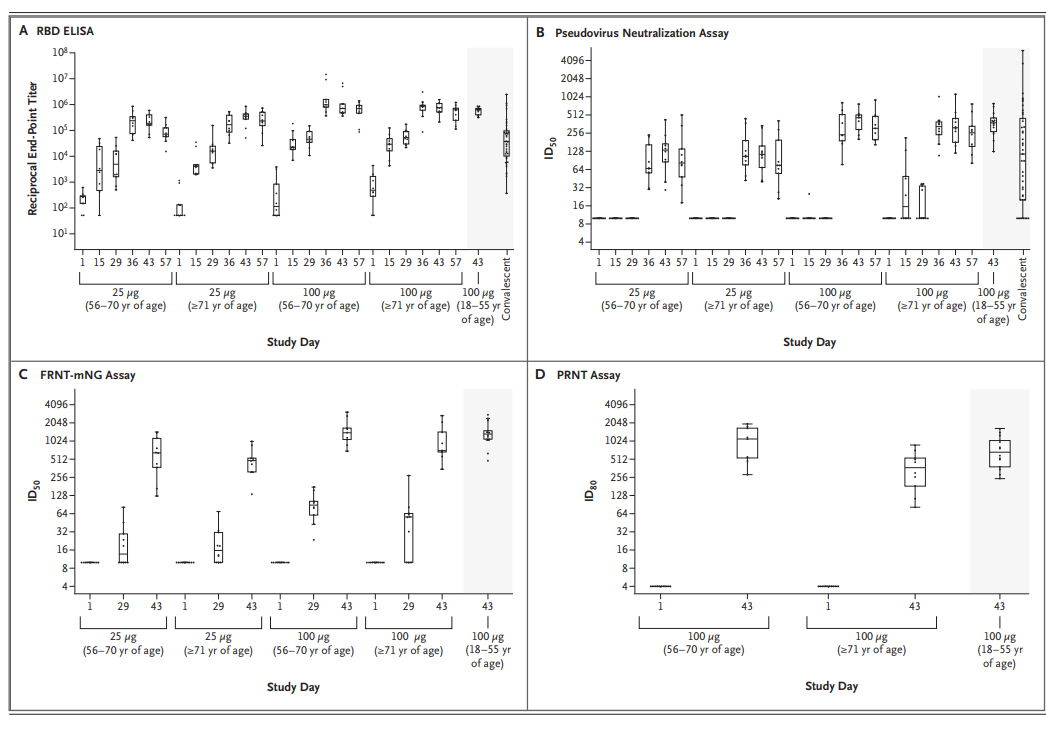
Last week, Moderna released the results of the expansion of their phase 1 trial of mRNA-1273, one of the more prominent COVID vaccine candidates in trials, which included the preliminary safety and immunogenicity data for healthy participants who were 56 years of age or older (Table 1). The trial followed similar dosing and antibody response analysis protocols from their previous clinical trial in younger adults. The article, which was released in the New England Journal of Medicine (link here), reported the vaccine induced high levels of both binding and neutralizing antibodies in participants (Figure 1). Moreover, the time- and dose-dependent trends were similar to those seen in their vaccine trial in younger adults, with no systematic differences in binding-antibody responses being observed between the two older-age subgroups and the younger population. Correspondingly, the responses after the second vaccination were similar to responses observed in patients who had recovered from Covid-19 and had donated convalescent serum.
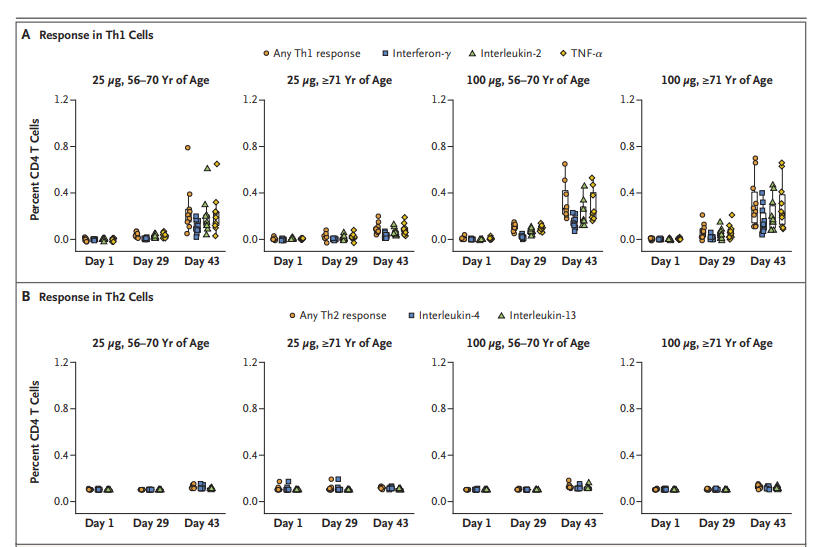
Interestingly, the vaccine also elicited a strong CD4 cytokine response involving type 1 helper T (Th1) cells among participants (Figure 2), similar to results observed in the younger subgroup. Another noteworthy aspect of this small trial was that there were no reports of serious adverse effects in this cohort of older participants, a group that is particularly at risk for illness and death from Covid-19. Those that experienced side effects described them as mostly mild to moderate with the most common being headaches, fatigue, myalgia, chills, and injection-site pain.
As we near the end of the year, multiple vaccine trials are promising to deliver a candidate that will help mitigate the incidence and mortality rates of the ongoing pandemic. As we move forward, researchers need to ensure that proper investigational protocols are being developed, and followed, to deliver a vaccine product that is both safe and effective for different subgroups of the public, at different ages, genders, ethnicities, and people with different comorbidities . As vaccines become approved and manufactured, we need to ensure that those groups at most risk (like those in this trial) receive the first round of immunizations!
References
Anderson et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. New England Journal of Medicine DOI: 10.1056/NEJMoa2028436 (2020).
Leave a Reply