Deoxysugars are often found as structural components of natural products, and have been shown in many cases to be important for the biological activities of the parent compounds. The study of the biosynthesis of unusual sugars and their transfer to acceptor molecules is critical to understanding and controlling the biological processes mediated by sugars. Over the years, our group has pioneered studies of the biosynthesis of deoxysugars. We use combined genetic, biochemical, and chemical methods to elucidate the individual steps in a number of deoxysugar biosynthetic pathways, and in the process, have identified many mechanistically interesting enzymatic transformations. We also work on exploring the substrate specificities of deoxysugar biosynthetic enzymes as a means to synthesize a variety of deoxysugars in vivo and in vitro. Our current efforts focus on the elucidation of the mechanisms of novel enzymes in deoxysugar biosynthetic pathways, characterization of macrolide glycosyltransferases, and construction of engineered sugar biosynthetic pathways to produce new glycosylated natural products, which can potentially be used to combat antibiotic resistant pathogenic bacterial strains. The deoxysugar biosynthetic pathways presently under investigation include those for desosamine (1), mycaminose (2), mycarose, forosamine, digitoxose, and kijanose.
Glycosyltransferases (GTs) are the enzymes catalyzing the attachment of sugars to acceptor substrates. Previous genetic studies performed by my group have shown that the macrolide GT, DesVII, from the methymycin (3)/neomethymycin (4) biosynthetic pathway of Streptomyces venezuelae, is substrate flexible in vivo, accepting many sugar intermediates that accumulate in deoxysugar biosynthetic gene knockout mutants. More recently, the activity of DesVII was reconstituted in vitro, the first demonstration of in vitro activity of a macrolide GT. Interestingly, the activity of DesVII is highly dependent on the presence of a protein of previously unknown function, DesVIII (see Scheme 1, 1 → 3/4). In vivo work on two other macrolide GTs, TylM2 and MycB, has demonstrated that these GTs also require DesVIII homologs from their respective pathways for full activity. The details of this unprecedented requirement for activator proteins by these GTs are being studied in more detail in our group.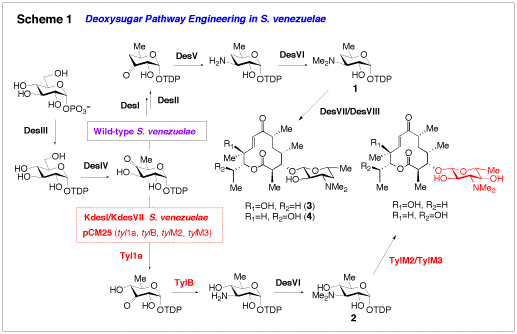
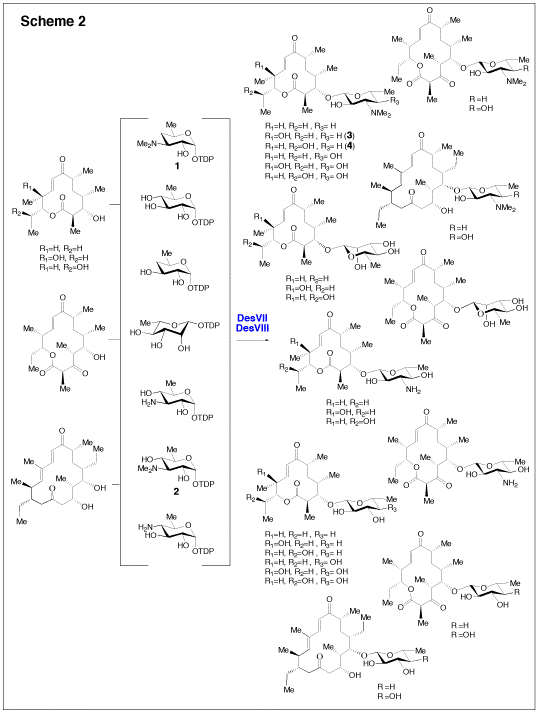
Because deoxysugars are critical in many cases for the activity of their parent compounds, changing the structure of these sugars holds potential to change the potency or specificity of the bioactivity of the glycosylated compounds. Toward this goal, we have initiated an effort to apply our cumulative knowledge of deoxysugar biosynthesis and glycosyltransfer in antibiotic biosynthetic pathways to generate novel antibiotic analogues in vivo and in vitro. This concept is termed glycodiversification. We have demonstrated in vivo that disruption of deoxysugar biosynthetic genes in the methymycin producer, S. venezuelae (desI and desVII disruption mutant, see Scheme 1) followed by heterologous expression of sugar biosynthetic genes and GTs from other pathways (such as tyl1a, tylB, tylM2 andtylM3 from the mycaminose pathway, see Scheme 1) can be used to generate macrolide derivatives with designed sugar structures. This method is being explored to construct collections of engineered strains which would produce libraries of new macrolide antibiotics. In parallel, a small library of sugar substrates were recently prepared chemoenzymatically and tested as substrates of DesVII/DesVIII in vitro, yielding a collection of new glycosylated macrolides, and further demonstrating the utility of DesVII as a highly substrate flexible GT (Scheme 2).